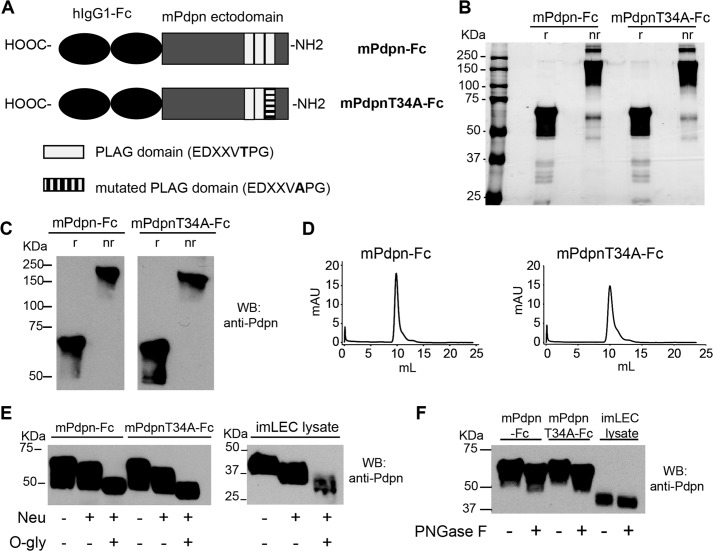
FIGURE 1.
Characterization of two recombinant mouse podoplanin Fc chimera isoforms, mPdpn-Fc and mPdpnT34A-Fc. A, schematic representation of mPdpn-Fc and mPdpnT34A-Fc. B, recombinant mPdpn-Fc isoforms were produced in CHO cells, affinity purified using Protein A and samples were resolved by SDS-PAGE under reducing (r) or non-reducing (nr) conditions. Silver staining revealed that both proteins were >95% pure. C, both isoforms were stained by an antibody directed against mouse podoplanin in Western blot and were present as disulfide-linked dimers. D, purified proteins were analyzed by gel filtration chromatography on a Superdex 200 10/30 column. They eluted as single species. E, to characterize their glycosylation, purified mPdpn-Fc and mPdpnT34A-Fc as well as the cell lysate of mouse immortalized LEC (imLEC) were digested with neuraminidase (Neu) followed by O-glycosidase (O-gly). F, to test for the presence of N-glycans, digestion was performed with peptide N-glycanase F (PNGase F). Samples were resolved by SDS-PAGE and immunoblotted with an anti-mouse Pdpn antibody (E and F). Both isoforms were sialylated and decorated with core-1 O-glycans. Moreover, the extent of sialylation and O-glycosylation was comparable with one of the endogenous podoplanin expressed by imLEC. The recombinant proteins and endogenous Pdpn appeared to also be N-glycosylated.