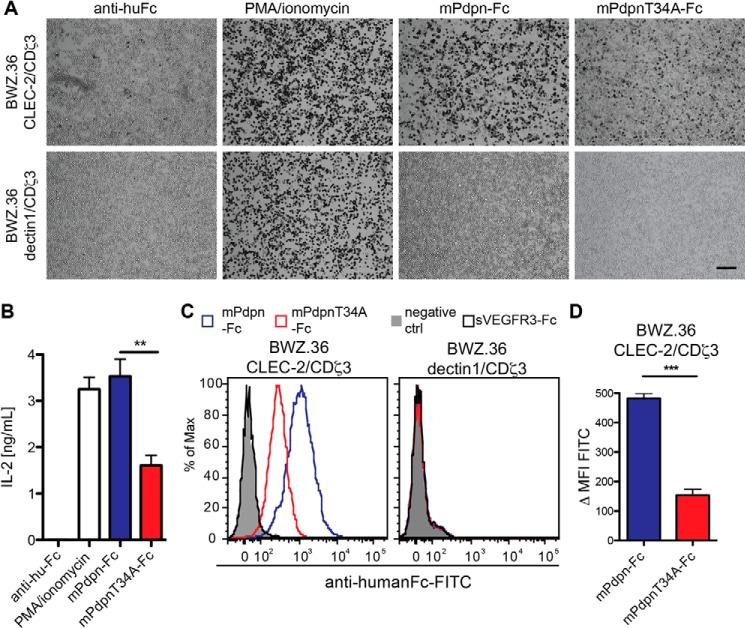
FIGURE 2.
mPdpnT34A-Fc showed a reduced ability to bind to and activate CLEC-2 reporter cells. BWZ.36 CLEC-2/CDζ3 and dectin1/CDζ3 reporter cells were used. Binding of a specific ligand to the C-type lectin-like receptors (CLEC-2 or dectin1) results in β-galactosidase expression and IL-2 production. A, CLEC-2 and dectin1 reporter cells were added to the mPdpn-Fc isoforms (20 ng/ml) immobilized through a human Fc capturing antibody or to the human Fc capturing antibody only. Reporter cells cultured with phorbol 12-myristate 13-acetate (PMA)-ionomycin served as a positive control of cell activation. Pictures were taken with an inverted microscope (×5 magnification). Scale bar, 200 μm. The number of X-gal-stained, activated CLEC-2 reporter cells was much lower on mPdpnT34A-Fc-coated than on mPdpn-Fc-coated plates. None of the immobilized proteins activated dectin1-expressing cells. B, IL-2 secreted by activated CLEC-2 reporter cells was measured by ELISA. Significantly less IL-2 was produced by the CLEC-2 reporter cells bound to mPdpnT34A-Fc as compared with mPdpn-Fc. C, CLEC-2 or dectin1-expressing cells were incubated with mPdpn-Fc isoforms, soluble VEGFR3-Fc (20 ng/ml), or buffer only (negative control). After incubation with a FITC-labeled, anti-human Fc antibody, binding was assessed by flow cytometry. D, Δ median fluorescent intensity (ΔMFI) was analyzed with FlowJo software. mPdpnT34A-Fc was found to bind less well to the CLEC-2-expressing cells compared with mPdpn-Fc. This binding was specific, as no binding was observed to the dectin1-expressing cells. Data represent mean ± S.E., statistical significance was analyzed using the unpaired Student's t test, **, p < 0.01; ***, p < 0.0001.