Abstract
Structural brain abnormalities identified at near-term age have been recognized as potential predictors of neurodevelopment in children born preterm. The aim of this study was to examine the relationship between neonatal physiological risk factors and early brain structure in very-low-birth-weight (VLBW) preterm infants using structural MRI and diffusion tensor imaging (DTI) at near-term age.
Structural brain MRI, diffusion-weighted scans, and neonatal physiological risk factors were analyzed in a cross-sectional sample of 102 VLBW preterm infants (BW ≤ 1500 g, gestational age (GA) ≤ 32 weeks), who were admitted to the Lucile Packard Children's Hospital, Stanford NICU and recruited to participate prior to routine near-term brain MRI conducted at 36.6 ± 1.8 weeks postmenstrual age (PMA) from 2010 to 2011; 66/102 also underwent a diffusion-weighted scan. Brain abnormalities were assessed qualitatively on structural MRI, and white matter (WM) microstructure was analyzed quantitatively on DTI in six subcortical regions defined by DiffeoMap neonatal brain atlas. Specific regions of interest included the genu and splenium of the corpus callosum, anterior and posterior limbs of the internal capsule, the thalamus, and the globus pallidus. Regional fractional anisotropy (FA) and mean diffusivity (MD) were calculated using DTI data and examined in relation to neonatal physiological risk factors including gestational age (GA), bronchopulmonary dysplasia (BPD), necrotizing enterocolitis (NEC), retinopathy of prematurity (ROP), and sepsis, as well as serum levels of C-reactive protein (CRP), glucose, albumin, and total bilirubin.
Brain abnormalities were observed on structural MRI in 38/102 infants including 35% of females and 40% of males. Infants with brain abnormalities observed on MRI had higher incidence of BPD (42% vs. 25%) and sepsis (21% vs. 6%) and higher mean and peak serum CRP levels, respectively, (0.64 vs. 0.34 mg/dL, p = .008; 1.57 vs. 0.67 mg/dL, p= .006) compared to those without. The number of signal abnormalities observed on structural MRI correlated to mean and peak CRP (rho = .316, p = .002; rho = .318, p= .002). The number of signal abnormalities observed on MRI correlated with thalamus MD (left: r= .382, p= .002; right: r= .400, p= .001), controlling for PMA-at-scan. Thalamus WM microstructure demonstrated the strongest associations with neonatal risk factors. Higher thalamus MD on the left and right, respectively, was associated with lower GA (r = −.322, p = .009; r= −.381, p= .002), lower mean albumin (r = −.276, p= .029; r= −.385, p= .002), and lower mean bilirubin (r = −.293, p= .020; r= −.337 p= .007).
Results suggest that at near-term age, thalamus WM microstructure may be particularly vulnerable to certain neonatal risk factors. Interactions between albumin, bilirubin, phototherapy, and brain development warrant further investigation. Identification of physiological risk factors associated with selective vulnerability of certain brain regions at near-term age may clarify the etiology of neurodevelopmental impairment and inform neuroprotective treatment for VLBW preterm infants.
Keywords: MRI, Diffusion tensor imaging, White matter microstructure, Brain development, Risk factors, Preterm infants
Abbreviations: VLBW, very-low-birth-weight; GA, gestational age; PMA, post-menstrual age; DTI, diffusion tensor imaging; FA, fractional anisotropy; MD, mean diffusivity; CC, corpus callosum; IC, internal capsule; ALIC, anterior limb of the internal capsule; PLIC, posterior limb of the internal capsule; GloP, globus pallidus
Highlights
-
•
Biomarkers of inflammation in preterm infants correlated with brain abnormalities detected on near-term structural MRI.
-
•
Biomarkers of inflammation in preterm infants correlated with near-term WM microstructure assessed on DTI.
-
•
Signal abnormalities observed on near-term structural MRI correlated with increased thalamus MD.
1. Introduction
Children born preterm with very-low-birth-weight (VLBW) are at higher risk for neurodevelopmental impairments, such as cerebral palsy, developmental delay, and possibly autism spectrum disorder (Woodward et al., 2012; Movsas et al., 2013). Brain abnormalities identified in the neonate may result in higher risk of neurologic impairment and later functional deficits (Woodward et al., 2012). Rapidly developing early brain regions may be selectively vulnerable to injury that may underlie adverse outcome (Johnston and Hoon, 2000; Miller and Ferriero, 2009; Liu et al., 2013). Previous studies have demonstrated widespread differences in white matter (WM) development and regional cortical volumes in infants born VLBW compared to age-matched full-term infants (Hüppi et al., 1998; Inder et al., 2005; Thompson et al., 2007; Lee et al., 2013; Thompson et al., 2013). These structural differences may be biomarkers for early brain injury and adverse outcome.
Near-term neuroimaging data can inform early prognosis for preterm infants and has potential to guide early intervention at a time of optimal neuroplasticity. Specifically, structural MRI can inform about gross brain injury, which has been shown to correlate with outcome (Miller and Ferriero, 2009; Liu et al., 2013); however, diffusion tensor imaging (DTI) of early WM regions may be a more sensitive biomarker of later neurodevelopment than structural MRI (Arzoumanian et al., 2003; Rose et al., 2007; Rose et al., 2009; De Bruïne et al., 2013). DTI quantifies the diffusion properties of tissues and is used to investigate WM microstructure. As the brain develops, water content decreases, extracellular spaces diminish, and cellular microstructures become more complex and organized; these changes can be quantified with DTI (Dubois et al., 2008; Lee et al., 2013; Dubois et al., 2013; Oishi et al., 2013; Nossin-Manor et al., 2013).
Scalars obtained from DTI can assess brain development and maturation and include fractional anisotropy (FA), mean diffusivity (MD), axial diffusivity (AD), and radial diffusivity (RD) (Pierpaoli et al., 1996). FA reflects the degree of diffusion anisotropy within a voxel, and is determined by fiber diameter and density, myelination, extracellular diffusion, inter-axonal spacing, and intra-voxel fiber-tract coherence. MD is a calculation of average diffusion along the three main axes, relative to the primary direction of diffusion, or AD. AD is thought to reflect fiber coherence and structure of axonal membranes. RD, the mean of diffusivities perpendicular to the primary axis, is thought to represent the degree of myelination (Song et al., 2002; Chen et al., 2011). In neonatal WM, FA has been found to increase with age, while MD, AD, and RD decrease with age, likely due to increased fiber organization, axonal coherence, and preliminary myelination (De Bruïne et al., 2013; Rose et al., 2014). Analyses of DTI scans obtained during the neonatal period may serve as potential predictors of neurodevelopment in children born preterm (Hüppi and Dubois, 2006; Rose et al., 2009; Thompson et al., 2013).
Previously, we reported DTI findings on regional brain microstructure at near-term age in 45 VLBW preterm infants with no evidence of gross brain abnormalities, a subpopulation of infants reported in the current study (Rose et al., 2014). WM microstructure was analyzed in 19 subcortical regions. Results indicated a general pattern of central to peripheral regional WM development and of posterior to anterior WM development within regions of the corona radiata, corpus callosum (CC) and internal capsule (IC). Projection fibers of the PLIC demonstrated the highest degree of WM maturation on DTI (Vassar et al., 2013; Rose et al., 2014). The ALIC was less mature but appeared to be one of the most rapidly developing WM regions at near-term age, consistent with early stages of myelination. White matter within the thalamus and globus pallidus (GP) demonstrated higher FA and lower MD values and had larger, less-variable volumes selected, than the putamen and caudate (Rose et al., 2014), suggesting that these regions may be more reliably assessed at near-term age. These findings, as well as other recent studies (Dubois et al., 2013), suggest that the developmental status of WM regions is an important consideration in the interpretation of neonatal neuroimaging studies, and informed the selection of regions analyzed in the current study.
Rapidly developing brain regions in the neonate may be selectively vulnerable to injury due to morbidities and clinical factors common during the neonatal period. Consequently, abnormal WM development in these regions may be useful for identifying infants with higher risk for adverse outcomes. Known risk factors for poor neurodevelopment following preterm birth include sepsis (Stoll et al., 2002; Schlapbach et al., 2011; Hentges et al., 2013; Mitha et al., 2013), bronchopulmonary dysplasia (Schmidt et al., 2003; Vohr et al., 2005; Karagianni et al., 2011; Schlapbach et al., 2012), necrotizing enterocolitis (NEC) (Hintz et al., 2005; van Vliet et al., 2013), and male sex (Wood et al., 2005; Vohr et al., 2005; Rose et al., 2009; Beaino et al., 2010; Morsing et al., 2011). The aim of this study was to examine the relationship between neonatal physiological risk factors and early brain microstructure in VLBW preterm infants at near-term age, based on qualitative analysis of structural MRI and on semi-automated, atlas-based DTI analysis.
2. Methods
2.1. Subjects
Near-term scans were obtained from 102 VLBW neonates, representing 76% of eligible participants treated at Lucile Packard Children's Hospital (LPCH) between 1/1/10 and 12/31/11. Parents were approached prior to scheduled MRI and consent was obtained for this IRB-approved study. Infants with GA-at-birth ≤32 weeks, birth weight ≤1500 g, and no evidence of genetic disorders or congenital brain abnormalities were included. Sixty-six of the 102 infants had successful DTI scans, collected at the end of routine near-term MRI scans, prior to discharge.
2.2. Neonatal risk factor definition and data collection
Demographic data and neonatal physiological risk factors were assessed in 102 VLBW preterm infants as recorded in the medical record. Demographics included gestational age (GA), birth weight (BW), sex, Apgar score at 5 minutes postnatal (Apgar-at-5 min), multiple births, maternal age, pregnancy-induced hypertension (PIH), and premature rupture of membranes. Physiological risk factors included the presence of bronchopulmonary dysplasia (BPD) defined as having a history of respiratory distress syndrome and treated with oxygen at 36 weeks PMA, sepsis confirmed with positive blood culture (Sood et al., 2012), necrotizing enterocolitis (NEC), and retinopathy of prematurity (ROP) at any time during hospital stay. Serum levels of mean and peak C-reactive protein (CRP), as well as mean glucose, albumin, and total bilirubin levels during the first 2-weeks of life were recorded. Laboratory values were obtained through a medical record search conducted with Stanford Center for Clinical Informatics and STRIDE (Stanford Translational Research Integrated Database Environment) (Lowe et al., 2009).
Neonatal demographic and physiological risk factors were selected for analysis based on their association with poor neurodevelopmental outcome in previous studies, as detailed in the Introduction. As noted in Table 1, serum levels of CRP, glucose, albumin, and total (T) bilirubin were sampled multiple times over the first two postnatal weeks. Other risk factors were identified based on physician report in the medical record.
Table 1.
Demographic and physiological risk factors.
| All Neonates n = 102 |
Normal MRI n = 64 |
Abnormal MRI n = 38 |
Neonates with DTI n = 66 |
|
|---|---|---|---|---|
| Males n (% with abnormal MRI) | 42 | 25 | 17 (40%) | 25 |
| Females n (%with abnormal MRI) | 60 | 39 | 21 (35%) | 41 |
| Gestational age (weeks) mean (SD) | 28.7 (2.4) | 28.9 (2.3) | 28.3 (2.6) | 28.9 (2.3) |
| Birth-weight (g) mean (SD) | 108 (279) | 1102 (276) | 1162 (285) | 109 (266) |
| PMA-at-scan (weeks) mean (SD) | 36.6 (1.8) | 36.5 (1.6) | 36.8 (2.0) | 36.5 (1.3) |
| Maternal age (years) mean (SD) | 31.6 (6.0) | 32.2 (5.4) | 30.6 (6.8) | 31.9 (6.1) |
| Multiple births mean (SD) | 1.75 (.95) | 1.8 (.88) | 1.7 (1.1) | 1.8 (.92) |
| Apgar at-5-min mean (SD) | 7.4 (1.8) | 7.7 (1.6) | 6.8 (2.2) | 7.4 (1.7) |
| Days on ventilation mean (SD) | 11.1 (18.4) | 7.7 (13.9) | 16.7 (23.2) | 8.7 (14.4) |
| Bronchopulmonary dysplasia, n (%) | 32 (31%) | 16 (25%) | 16 (42%) | 18 (27%) |
| Necrotizing enterocolitis, n (%) | 14 (14%) | 8 (13%) | 6 (16%) | 7 (11%) |
| Retinopathy of prematurity, n (%) | 72 (71%) | 40 (63%) | 32 (84%) | 45 (68%) |
| Sepsis a, n (%) | 12 (12%) | 4 (6%) | 8 (21%) | 59 (9%) |
| Mean CRPb, mg/dL mean (SD) | .45 (.58) | .34 (.45) | .64 (.72)f | .38 (.49) |
| p = .008 | ||||
| Peak CRPb, mg/dL mean (SD) | 1.00 (1.49) | .67 (.98) | 1.57 (1.99)f | .83 (1.30) |
| p = .006 | ||||
| Mean glucosec, mg/dL mean (SD) | 103.1 (20.9) | 101.8 (19.3) | 105.2 (23.7) | 103.0 (18.3) |
| p = .488 | ||||
| Mean albumind, mg/dL, mean (SD) | 2.4 (.3) | 2.4 (0.3) | 2.4 (0.4) | 2.4 (.3) |
| p = .185 | ||||
| Mean T bilirubine mg/dL, mean (SD) | 5.4 (1.3) | 5.4 (1.3) | 5.4 (1.4) | 5.3 (1.3) |
| p = .802 | ||||
Confirmed with a positive blood culture.
CRP sampled an average of 3.72 (1.9) times in the first 2-weeks.
Glucose sampled an average of 13.6 (5.8) times in the first 2-weeks.
Albumin sampled 9.7 (6.1) times in the first 2-weeks.
Total bilirubin sampled 13.6 (2.9) times in the first 2-weeks.
Significantdifference between infants with normal versus abnormal MRI assessed with Mann–Whitney U using two-tailed significance of p < .01 for serum CRP, glucose, albumin, and bilirubin.
2.3. MRI data acquisition
Brain MRI scans were performed on 3T MRI (GE-Discovery MR750, GE 8-Channel HD head coil) at Lucile Packard Children's Hospital at Stanford University. The MRI included T1-weighted, T2-weighted, and diffusion-weighted imaging (DWI) and diffusion tensor imaging (DTI) scans. A gradient echo 3-plane localizer was used, and an asset calibration was proscribed to utilize parallel imaging. From the 3-plane localizer, T1 images were generated with the following parameters: TE = 9l.0, TR = 2200, FOV = 20 cm, matrix size = 320 × 224, slice thickness 3.0 × 0.5 mm spacing, NEX = 1. T2, DWI, and DTI axial scans were proscribed using a single acquisition, full phase field of view (FOV). The Fast Recovery, Fast Spin Echo T2 scan imaging parameters were as follows: TE = 85 ms, TR = 2500, FOV = 20 cm, matrix size = 384 × 224; slice thickness = 4.0 mm × 0.0 mm spacing. Axial T2 FLAIR parameters were as follows: TE = 140, TR = 9500, FOV = 20 cm, slice thickness = 4.0 mm × 0.0 mm, inversion time = 2300, fluid attenuated inversion recovery matrix = 384 × 224. Axial DWI parameters were as follows: TE = 88.8, TR = 10,000, FOV = 20 cm, slice thickness = 4.0 mm × 0.0 mm spacing, matrix = 128 × 128. The DTI scan was based on a diffusion-weighted, single-shot, spin-echo, echo-planar imaging sequence with a slice thickness of 3.0 mm, a matrix size of 128 × 128, a 90° flip angle, FOV = 20 cm, TE = 88.8 ms, TR = 8000, (b = 1000 s/mm2). Diffusion was measured along 25 directions, with three B0 images. Two repetitions were obtained from 64/66 subjects. MRI scans were performed as the routine near-term neuroimaging for preterm infants and the diffusion-weighted sequences were collected at the end of these ˜25-minute MRI scans. Infants were swaddled and fed and typically remained asleep for the duration of the scan. Sedation was not utilized as part of the research protocol and typically was not utilized for routine near-term MRI, although it may have been prescribed according to clinical needs in some cases.
2.4. Radiological assessment
Brain abnormalities were assessed on structural MRI for degree of abnormality on a scale that defines categories of 1–5, as described in Table 2, and for the number of signal abnormalities observed on T1 and T2 scans in cerebral, intraventricular, internal capsule, basal ganglia, thalamus, and cerebellar regions. Radiological evaluation was performed by an experienced pediatric neuroradiologist, X.S., who was masked to all other participant data. Brain abnormalities identified in the research assessment were consistent with findings of brain abnormalities reported by the clinical neuroradiologist in the patient's medical record.
Table 2.
Categories used to classify structural MRI findings.
| Category | Radiological findings on MRI |
|---|---|
| C1 | No abnormality. |
| C2 | Minimal abnormality, such as subependymal hemorrhage or mineralization (e.g. sequelae of germinal matrix hemorrhage) with or without mild ventriculomegaly, with or without mild IVH. |
| C3 | Moderate to severe ventriculomegaly, with or without IVH |
| C4 | Parenchymal abnormality, including evidence of gray and/or white matter injury due to hemorrhage or ischemia, with or without IVH. |
| C5 | Congenital malformation (excluded from further analyses) |
2.5. DTI processing
In a subpopulation of 66 infants, semi-automated, atlas-based DTI was analyzed. Diffusion-weighted images were pre-processed using DTI-Studio based on parameters outlined in Jiang et al. (2006) and Oishi et al. (2011). For infants with two repetitions (n = 64/66), the best repetition was selected to eliminate images with artifacts or evidence of motion. If no full repetition was usable, a composite repetition was generated based on best image slices (provided the two repetitions were well-registered). Automated Image Registration was performed using an affine transformation to further correct for eddy current distortions and minor head motion.
Each brain image was skull-stripped with ROI Editor using B0 and trace images to distinguish brain matter from intracranial CSF and skull, and manually rotated to align with the JHU neonatal template image. DTI images were processed with DiffeoMap (http://www.mristudio.org) using FA and trace (the sum of the 3 orthogonal diffusivities per voxel) maps to perform a large deformation diffeomorphic metric mapping (LDDMM) transformation (Oishi et al., 2011). Each brain was normalized to map onto the neonatal atlas and segmented into 126 regions. Based on the trace map, regions >0.006 mm2/s were considered CSF and were excluded from the segmented regions. A threshold of FA >0.15 was then applied to segment developing WM tracts (Vassar et al., 2013). Regions selected within the FA >0.15 threshold were then mapped onto the FA and trace maps, and regional values were obtained. MD values were defined as an average of the three directions of diffusion calculated from the trace image.
We examined subcortical WM defined by the JHU parcellation atlas that was well-visualized with near-term MRI and DTI, and known to mediate motor or cognitive function, as illustrated in Fig. 1. Specifically, we measured FA and MD in commissural fibers of the genu and splenium of the ccs, projection fibers of anterior and posterior limbs of the internal capsule (ALIC and PLIC respectively) and in the WM of subcortical nuclei of the thalamus and globus pallidus (GloP).
Fig. 1.
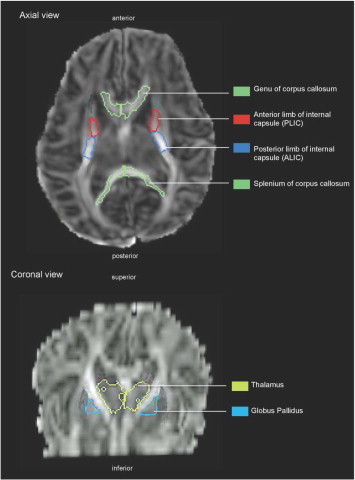
Brain regions selected in a representative infant DTI scan using JHU neonatal atlas, axial and corona views.
These regions were selected for analysis based on prior work that demonstrated that these regions were reliably detected by semi-automated, atlas based analysis of DTI at near-term age (Rose et al., 2014) based on percent volume above the FA >0.15 threshold, relative to original volumes identified by the neonatal atlas, as applied to the 45 infants with no evidence of abnormalities on MRI and scanned prior to 40 weeks PMA (Vassar et al., 2013).
2.6. Statistical analysis
Neonatal demographic and physiological risk factors of GA, CRP, glucose, albumin, and bilirubin were compared in infants with and without brain abnormalities observed on MRI using nonparametric Mann–Whitney U, with a significance level of p< .01 as a correction for multiple comparisons (Table 1). The Spearman’s correlation was used to assess associations between neonatal risk factors and brain MRI score and the number of signal abnormalities observed on MRI with a significance level of p< .008, to correct for multiple comparisons (Table 3). Partial correlations were reported between MRI score and number of signal abnormalities observed on MRI and MD values, after controlling for PMA-at-scan with a significance level of p< .01 as a correction for multiple comparisons. Partial correlations were reported between neonatal risk factors and MD values in the CC, ALIC, PLIC, thalamus, and GloP, after controlling for PMA-at-scan with significance level of p < .01—corrected for multiple comparisons (Table 4). Differences between male and female MD values, corrected for PMA-at-scan, were compared using the nonparametric Mann–Whitney test, with a significance level of p < .05. Spearman's correlation was used to assess associations among risk factors with continuous values with a significance level of p< .01 (Table 5). The variance inflation factors (VIF) were estimated as a diagnostic for co-linearity between risk factors. Statistical analysis was performed using SPSS Statistics software Version 21 (IBM).
Table 3.
Correlations between neonatal risk factors and brain MRI score and number of signal abnormalities on MRI at near-term age.
| Neonatal risk factors | MRI score Range = 1–4 Mean (SD) = 2.2 (1.0) |
# of signal abnormalities Range = 0–13 Mean (SD) = 2.1 (3.0) |
|
|---|---|---|---|
| Gestational age at birth | Rho | −.140 | −.197 |
| Sig. | p = .163 | p = .048 | |
| Apgar at 5 min | Rho | −.236 | −.303 |
| Sig. | p = .031 | p= .005a | |
| C-reactive protein | Rho | .220 | .316 |
| Sig. | p = .031 | p= .002a | |
| Peak C-reactive protein | Rho | .211 | .318 |
| Sig. | p = .039 | p= .002a | |
| Glucose | Rho | −.001 | −.028 |
| Sig. | p = .994 | p = .787 | |
| Albumin | Rho | − .170 | −.193 |
| Sig. | p = .095 | p = .058 | |
| Total bilirubin | Rho | −.077 | −.065 |
| Sig. | p = .452 | p = .528 | |
Spearman’s rho, two-tailed significance p < .008.
Table 4.
Correlations between neonatal risk factors and near-term regional brain white matter microstructure mean diffusivity (MD), corrected for PMA-at-scan, are reported for the genu, splenium (Splen), posterior limb of internal capsule (PLIC), anterior limb of internal capsule (ALIC), thalamus (Thal), and globus pallidus, (GloP). Risk factors include gestational age at birth (GA), mean and peak serum C-reactive protein (CRP), and mean serum glucose, albumin, and total (T) bilirubin.
| Genu MD |
Splen MD |
L ALIC, MD |
R ALIC, MD |
L PLIC, MD |
R PLIC, MD |
L Thal, MD |
R Thal, MD |
L GloP, MD |
R GloP, MD | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| GA (N = 66) | r | .116 | −.176 | .160 | .065 | .067 | .033 | −.322a | −.381a | −.088 | −.144 |
| Sig. | .359 | .160 | .203 | .608 | .596 | .794 | .009 | .002 | .485 | .252 | |
| CRP (N = 63) | r | .189 | −.152 | −.025 | −.229 | .008 | −.128 | −.125 | −.125 | .000 | −.040 |
| Sig. | .141 | .239 | .847 | .073 | .954 | .321 | .334 | .331 | .999 | 0.757 | |
| Peak CRP (N = 63) | r | .234 | −168 | .028 | −.158 | .010 | −.120 | −.075 | −.057 | −.039 | −.024 |
| Sig. | .068 | .191 | .832 | .221 | .941 | .355 | .564 | .661 | .763 | .852 | |
| Glucose (N = 64) | r | −.170 | .028 | −.178 | −.104 | −.175 | −.189 | .043 | .130 | −.092 | −.038 |
| Sig. | .183 | .825 | .163 | .415 | .170 | .138 | .740 | .310 | .474 | .769 | |
| Albumin (n = 64) | r | .036 | −.004 | −.059 | −.136 | −.149 | −.106 | −.276 | −.385a | −.141 | −.076 |
| Sig. | .780 | .975 | .645 | .286 | .244 | .408 | .029 | .002 | .272 | .551 | |
| T Bilirubin (n = 64) | r | .111 | −.095 | −.006 | −.105 | −.023 | −.074 | −.293 | −.337a | −.145 | −.196 |
| Sig. | .387 | .461 | .963 | .411 | .861 | .566 | .020 | .007 | .257 | .123 |
Partial correlation (r), two-tailed significance p < .01.
Table 5.
Correlations among neonatal risk factors. Risk factors include gestational age at birth (GA), mean and peak serum C-reactive protein (CRP), and mean serum glucose, albumin, and total (T) bilirubin.
| GA | CRP | Peak CRP | Glucose | Albumin | Bilirubin | ||
|---|---|---|---|---|---|---|---|
| GA | Rho | 1 | −.196 | −.258 | −.553a | .573a | .647a |
| Sig. | .055 | .011 | .000 | .000 | .000 | ||
| N | 102 | 97 | 97 | 98 | 98 | 98 | |
| CRP | Rho | −.196 | 1 | .973a | .033 | −.338a | −.262a |
| Sig. | .055 | .000 | 0.752 | .001 | .010 | ||
| N | 97 | 97 | 97 | 97 | 97 | 97 | |
| Peak CRP | Rho | −.258 | .973a | 1 | .083 | −.398a | −.320a |
| Sig. | .011 | .000 | .420 | .000 | .001 | ||
| N | 97 | 97 | 97 | 97 | 97 | 97 | |
| Glucose | Rho | −.553a | .033 | .083 | 1 | −.363a | −.433a |
| Sig. | .000 | .752 | .420 | .000 | .000 | ||
| N | 98 | 97 | 97 | 98 | 98 | 98 | |
| Albumin | Rho | .573a | −.338a | −.398a | −.363a | 1 | .609a |
| Sig. | .000 | .001 | .000 | .000 | .000 | ||
| N | 98 | 97 | 97 | 98 | 98 | 98 | |
| Total bilirubin | Rho | .647a | −.262 | −.320a | −.433a | .609a | 1 |
| Sig. | .000 | .010 | .001 | .000 | .000 | ||
| N | 98 | 97 | 97 | 98 | 98 | 98 | |
Spearman’s correlation (rho), two-tailed significance p < .01.
3. Results
Demographic characteristics and physiological risk factors of all participants who had MRI (n = 102) and the subset population that also had DTI (n= 66) are reported in Table 1. Demographic data of the total population was similar to that of the sub-population who had both MRI and DTI. Demographic and physiological risk-factor data for participants with and without brain abnormalities observed on MRI are reported in Table 1. Brain abnormalities were identified on MRI in 38 out of 102 participants (37%), including 21/60 (35%) females and 17/42 (41%) males. Gestational age and BW were similar in those with and without brain abnormalities on MRI. Infants with brain abnormalities observed on MRI had higher incidence of BPD (42% vs. 25%) and sepsis (21% vs. 6%) and higher mean and peak serum CRP levels (0.64 vs. 0.34 mg/dL, p= .008; 1.57 vs. 0.67 mg/dL, p = .006) compared to those without brain abnormalities observed on MRI.
The correlations between neonatal risk factors are shown in Table 5. Of note, a strong association was found between mean bilirubin and BW (rho = .789, p = .000) and between mean albumin and mean bilirubin levels (rho = .604, p = .000). For risk factors with continuous values we estimated the variance inflation factors (VIF) as a diagnostic for collinearity and found that mean and peak values of CRP were collinear (VIF ≤ 9.9). Otherwise, among the mean values of risk factors we found no other collinearities, indicating that these mean values provide reasonably independent information for predictive models. The VIF ranged from 1.0 to 3.2, and fell below 10, the conservative criterion for indicating presence of collinearity.
Table 3 lists the mean and range of MRI score and the number of signal abnormalities observed on MRI, as well as correlations with physiological risk factors. The higher number of signal abnormalities was correlated to lower scores for Apgar-at-5-minutes and higher mean and peak CRP (r = .316, p= .002; r= .318, p= .002).
DTI analysis was performed on the selected brain regions shown in Fig. 1 as defined by the JHU neonatal atlas. Detection of brain abnormalities observed qualitatively on structural MRI compared to DTI findings indicated that the number of signal abnormalities observed on MRI was associated with thalamus MD (left: r= .382, p = .002; right: r= .400, p = .001), with trends for genu, splenium, ALIC, and PLIC MD. No associations were found between signal abnormalities observed on MRI and GloP MD.
No associations were found between neonatal risk factors and regional FA values, after controlling for PMA-at-scan.
Genu MD values, corrected for PMA-at-scan, were higher in males compared to females (p = .006). No other region demonstrated significant differences between males and females.
Table 4 lists associations between neonatal risk factors and MD values of the genu, splenium, PLIC, ALIC, thalamus, and GloP, controlled for PMA-at-scan. No significant association was found between regional MD values and serum CRP levels, only a trend of association between genu MD and peak CRP values was found (r= .234, p = .068). Thalamus MD demonstrated the strongest associations with neonatal risk factors, higher thalamus MD was associated with lower GA (left: r= −.322, p = .009; right: r= −.381, p= .002), lower mean serum albumin (left: r= −.276, p = .029; right: r= −.385, p= .002), and lower mean total bilirubin (left: r= −.293, p = .020; right: r= −.337 p= .007).
4. Discussion
This study examined the relationship between neonatal physiological risk factors and early brain structure that was observed on MRI and quantified using DTI. We found that more than one-third of infants had brain abnormalities observed on structural MRI. Infants with brain abnormalities on MRI demonstrated a similar degree of prematurity, however they had a higher incidence of BPD and sepsis and higher levels of serum CRP over the first 2 weeks of life, compared to those with no brain abnormalities on MRI. Evidence from DTI indicated that the thalamus WM microstructure was most strongly associated with signal abnormalities on structural MRI and with neonatal risk factors.
Advances in neonatal medicine have increased survival rates and improved outcome among VLBW preterm infants. The increased survival of VLBW preterm infants is associated with increased rate of neurodevelopmental impairment, which is currently 3–4 times that of the general population (Williams et al., 2010; Spittle et al., 2011). The incidence of CP in VLBW preterm infants is estimated to be 10–15% (Litt et al., 2005; Williams et al., 2010; Rose et al., 2009; Woodward et al., 2012), and is defined as “a group of disorders affecting the development of movement and posture, attributed to non-progressive disturbances to the developing fetal or infant brain” (Bax et al., 2005; Rosenbaum et al., 2007; CDC, 2013). Developmental coordination disorder may affect another 40% of VLBW preterm children of school age (Woodward et al., 2012). Cognitive impairment and/or language delays have been reported in 20–40% of school-age children born VLBW (Anderson, 2003; Litt et al., 2005; Hack, 2006; Larroque et al., 2008; Pritchard et al., 2009; Woodward et al., 2009; Aarnoudse-Moens et al., 2009; Ritter et al., 2014) and it is estimated that half of these children will require special educational assistance, (Wocadlo and Rieger, 2006; Larroque et al., 2008; Johnson et al., 2009; Delobel-Ayoub et al., 2009; Iwata et al., 2012). It is not currently possible to predict at near-term age which children will experience these physical and cognitive impairments, limiting our ability to apply early interventions during critical periods of development.
Brain MRI scans are commonly performed at near-term age in very preterm infants, and was envisioned as a potential opportunity to provide early prognosis in preterm infants. However, prognosis based on structural brain MRI has been of limited success in predicting risk for neurodevelopmental problems such as motor deficits, cognitive delay, and language impairment later in life (Kidokoro et al., 2011; Benini et al., 2013). DTI of early WM regions may be a more sensitive biomarker of later neurodevelopment than structural MRI (Arzoumanian et al., 2003; Rose et al., 2007; Rose et al., 2009; De Bruïne et al., 2013). Semi-automated, atlas-based DTI analysis has potential for clinical implementation and therefore was used in the current study. Diffusion was analyzed in WM regions which were previously suggested to be reliably detected by semi-automated, atlas based analysis of DTI at near-term age (Rose et al., 2014) and also mediate motor and cognitive function, domains known to be affected by preterm birth.
4.1. Neonatal risk factors and brain abnormalities observed on structural MRI
Brain abnormalities were identified in 38/102 infants, including 21/60 females (35%) and 17/42 males (40%). No differences in GA or BW were found, and while infants with brain abnormalities observed on MRI had lower Apgar scores at 5 min after birth, the trend did not reach significance. The value of Apgar scores for prediction of outcome has been debated, but Lie et al. (2010) found a strong correlation between low Apgar scores 5 min after birth and diagnosis of CP before age 5 in children with both low and normal BW. The incidence of BPD was slightly higher in those with brain abnormalities observed on MRI (41%) compared to those without (37%) but did not correlate with MRI score or the number of signal abnormalities on MRI. Bronchopulmonary dysplasia continues to be one of the main risk factors for motor impairments following preterm birth (Schmidt et al., 2003; Vohr et al., 2005; Karagianni et al., 2011; Schlapbach et al., 2012).
Infants with brain abnormalities on MRI had significantly higher mean and peak serum CRP levels compared to those with no brain abnormalities on MRI. Furthermore, number of signal abnormalities observed on MRI correlated to serum CRP levels. CRP is a plasma protein involved in inflammatory response via rapid response to cell death or injury; simultaneously production of other plasma proteins including albumin, decreases, thus, CRP levels have been proposed as a biomarker for sepsis and general inflammatory response in neonates (Carlo et al., 2011; Meem et al., 2011; Fan and Yu, 2012). One challenge is determining an optimal cutoff value for CRP levels for sensitive and specific identification of neonatal sepsis (Abdollahi et al., 2012; Boonkasidecha et al., 2013). Using a combination of biomarkers may hold more diagnostic accuracy than any one biomarker (Wang et al., 2013). Sepsis is a known risk factor for poor neurodevelopmental prognosis and cerebral palsy among VLBW preterm infants (Stoll et al., 2002; Schlapbach et al., 2011; Hentges et al., 2013; Mitha et al., 2013; van Vliet et al., 2013). Thus, the observed brain abnormalities may be related to inflammatory processes that occurred in the neonatal period.
Abnormal serum concentration of glucose, hormones, and proteins are commonly observed in VLBW preterm infants. Specifically, hypoglycemia was shown to be correlated with increased morbidity and mortality (Kao et al., 2006; Hays et al., 2006), as well as poor growth (Ramel et al., 2013) in VLBW infants. We did not find significant differences between serum levels of glucose, albumin, or bilirubin in those with or without brain abnormalities observed on MRI. Bilirubin levels observed in our population of neonates were not grossly abnormal, and thus our assessment of the role of these risk factors in brain development is limited.
4.2. Neonatal risk factors and WM microstructure on DTI
4.2.1. Corpus callosum
Diffusion imaging scalars were analyzed within association fibers of genu and splenium of the ccs, regions that are in preliminary stages of myelination at near-term age (Brody et al., 1987; Kinney et al., 1988; Provenzale et al., 2012). The posterior aspects (splenium) of the ccs were previously found to develop earlier than the anterior (genu) aspects (Vassar et al., 2013; Rose et al., 2014). Previous studies also reported that WM structure in the near–term CC may have prognostic value for future motor function (Counsell et al., 2008; Thompson et al., 2012). Males were found to have significantly higher genu MD, corrected for PMA-at-scan. The limited data on sexual dimorphism of neonatal CC development suggests that males may have slightly smaller or less developed CC at near-term age compared to females (Rose et al., 2009; Thompson et al., 2012). Male gender also was found to be associated with increased risk of adverse outcomes including CP and cognitive deficits following preterm birth (Wood et al., 2005; Vohr et al., 2005; Rose et al., 2009; Beaino et al., 2010; Morsing et al., 2011). Further investigation is needed to explain the physiological and neurological risk factors that increase morbidity among VLBW males.
4.2.2. Internal capsule
Diffusion was also analyzed in the projection fibers of the anterior and posterior limbs of the internal capsule. The PLIC includes motor fibers of the cortical spinal tract (CST) and has been found to be relatively more developed than the ALIC at near-term age (Vassar et al., 2013; Rose et al., 2014). Based on the work of Yakovlev and Lecours (1967) and Brody et al. (1987), regions of the CST are believed to be undergoing myelination during the near-term period. The CST descends through the posterior third of the PLIC and, consistent with this neuroanatomy, analyses of PLIC DTI at near-term age suggest that it may hold prognostic value for future motor development (Arzoumanian et al., 2003; Rose et al., 2007; Rose et al., 2009; De Bruïne et al., 2013). No further associations between neonatal risk factors and PLIC MD were found. This may be explained by the developmental trajectory of the PLIC, which is thought to be particularly vulnerable at the end of the second trimester, when developing vasculature and early myelination processes may increase vulnerability to WM injury (Hüppi et al., 1998; Johnston and Hoon, 2000; Neil et al., 2002; Miller and Ferriero, 2009; Liu et al., 2013).
4.2.3. Thalamus and globus pallidus
We found that thalamus WM microstructure demonstrated the strongest associations with neonatal risk factors, including GA, albumin, and bilirubin. Among VLBW neonates, GA has been found to be inversely correlated with cognitive and motor function later in childhood in several large studies (Bhutta et al., 2002; Constantinou et al., 2005; Odding et al., 2006; Aarnoudse-Moens et al., 2009; Woodward et al., 2009; Moore et al., 2012). Vulnerability of thalamus WM structure to neonatal risk factors could in part explain the impaired motor and cognitive function associated with preterm birth.
Two recent studies by Ball et al. observed decreased thalamus volume (Ball et al., 2012b) and impaired thalamocortical connectivity (Ball et al., 2012a) in infants born preterm, compared to age-matched term infants, suggesting that WM impairment may occur due to preterm birth. The thalamus is undergoing significant development and establishing cortical connections during the late second and third trimester of gestation (Kostović and Judas, 2010), and this process may be disrupted by preterm birth. The thalamus and basal ganglia (consisting of the caudate nucleus, putamen, and globus pallidus) are known to mediate motor processes and have been found to be vulnerable to hypoxic ischemic injury in infants (Cowan et al., 2003; Miller and Ferriero, 2009; Ball et al., 2012a; Nossin-Manor et al., 2012). Other investigators have also reported reduced volumes of these subcortical regions in preterm infants, compared to age-matched, term-born controls (Boardman et al., 2006; Srinivasan et al., 2007).
We found that higher thalamus MD was associated with lower mean albumin and with lower mean total bilirubin. Peak serum bilirubin during the first 2 weeks of life is known to be associated with increased risk of neurodevelopmental impairments (Oh et al., 2003; Oh et al., 2010). However, aggressive treatment with phototherapy may cause oxidative injury to cell membranes and increased mortality (Morris et al., 2008). Thus, the effect of high levels of bilirubin and aggressive bilirubin phototherapy on overall outcome among VLBW infants needs further investigation (Tyson et al., 2012), and is difficult to study independently (Hintz et al., 2011).
Recent studies suggest that unbound bilirubin is more informative than total serum bilirubin in prediction of neurodevelopmental trajectories in VLBW infants (Calligaris et al., 2007; Oh et al., 2010). The ratio of bound/unbound bilirubin is moderated by the availability of albumin, as bilirubin binds to albumin, preventing it from crossing the blood–brain barrier (Watchko, 2006; Bender et al., 2007). Thus, low albumin levels in the preterm infant may also be involved in the cytotoxicity of bilirubin and may hold prognostic value. Because unbound bilirubin is difficult to measure compared to total bilirubin, the ratio of total bilirubin/albumin may allow for estimation of exposure of the neonatal brain to unbound bilirubin (Sato et al., 2012).
The strong association found between albumin and total bilirubin levels in the current study suggests that low albumin levels may be primarily responsible for both increased thalamus MD and lower bilirubin levels. Phototherapy to reduce bilirubin levels is administered based on BW, and thus infants with lower BW will have lower bilirubin levels (Table 5). This may also contribute to the relationship between low bilirubin and high thalamus MD (Table 4). However, further study is required to examine the interactions between bilirubin, albumin, and sepsis and how they individually contribute to increased risk for neurodevelopment impairments. Furthermore, albumin levels are decreased as a result of inflammation, and ischemia-modified albumin levels may be used as prognostic tools for sepsis in neonates (Yerlikaya et al., 2014; Yakut et al., 2014).
5. Conclusion
This study examined the relationship between neonatal physiological risk factors and early brain structure in VLBW preterm infants. DTI was assessed to examine evidence of selective vulnerability of brain regions, which may ultimately serve to predict outcome and guide neuroprotective treatment for preterm infants. We used semi-automated atlas-based analysis of DTI because of its potential for clinical neuroimaging for neonates. We observed that the thalamus demonstrated the most associations with neonatal risk factors, and although these were low to moderate correlations, the findings may reflect selective vulnerability of the thalamus at near-term age. Limitations of this study include the cross-sectional design, the difficulty in studying risk factors independently, and the low incidence of some risk factors affecting brain development (e.g. NEC). Sequential neuroimaging during the near-term period would be helpful in revealing trajectories of development and how they relate to neonatal risk factors. Furthermore, correlation between clinical risk factors and regional brain structure and microstructure at near term age does not describe the relationship between brain abnormality and neurodevelopmental impairment. Results may have been influenced by accuracy of automated ROI generation, which is better for larger ROIs. As in any DTI investigation, results may also be influenced by differences in SNR between brain regions. Future studies using different diffusion-weighted analyses and scan sequences should be performed to further investigate WM structure in VLBW neonates and their relationship with neonatal physiological risk factors. Future studies may determine whether associations between neonatal risk factors and regional patterns of microstructural development could provide early prognostic biomarkers for future function that may ultimately guide early intervention aimed at reducing neurodevelopmental disabilities.
Acknowledgments
We wish to thank Dr. John Tamaresis for valuable statistical consultation. This research is supported in part by the NIH Clinical and Translational Science Award UL1 RR025744 for the Stanford Center for Clinical and Translational Education and Research (Spectrum) and for the Stanford Center for Clinical Informatics. STRIDE (Stanford Translational Research Integrated Database Environment) is a research and development project at Stanford University to create a standards-based informatics platform supporting clinical and translational research, and by the Lucile Packard Foundation for Children's Health. This material is based upon work supported by the National Science Foundation Graduate Research Fellowship under grant No. DGE-1147470.
References
- Aarnoudse-Moens C.S.H. Meta-analysis of neurobehavioral outcomes in very preterm and/or very low birth weight children. Pediatrics. 2009;124(2):717–728. doi: 10.1542/peds.2008-2816. 19651588 [DOI] [PubMed] [Google Scholar]
- Abdollahi A. Diagnostic value of simultaneous measurement of procalcitonin, interleukin-6 and hs-CRP in prediction of early-onset neonatal sepsis. Mediterranean Journal of Hematology and infectious Diseases. 2012;4(1) doi: 10.4084/MJHID.2012.028. 22708043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson P. Neurobehavioral outcomes of school-age children born extremely low birth weight or very preterm in the 1990s. JAMA: The Journal of the American Medical Association. 2003;289(24):3264–3272. doi: 10.1001/jama.289.24.3264. [DOI] [PubMed] [Google Scholar]
- Arzoumanian Y. Diffusion tensor brain imaging findings at term-equivalent age may predict neurologic abnormalities in low birth weight preterm infants. AJNR. American Journal of Neuroradiology. 2003;24(8):1646–1653. 13679287 [PMC free article] [PubMed] [Google Scholar]
- Ball G., Boardman J.P., Aljabar P. The influence of preterm birth on the developing thalamocortical connectome. Cortex; a journal devoted to the study of the nervous system and behavior. 2012 doi: 10.1016/j.cortex.2012.07.006. [DOI] [PubMed] [Google Scholar]
- Ball G. The effect of preterm birth on thalamic and cortical development. Cerebral Cortex (New York, N.Y.: 1991) 2012;22(5):1016–1024. doi: 10.1093/cercor/bhr176. 21772018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bax M. Proposed definition and classification of cerebral palsy, April 2005. Developmental Medicine and Child Neurology. 2005;47(8):571–576. doi: 10.1017/s001216220500112x. 16108461 [DOI] [PubMed] [Google Scholar]
- Beaino G. Predictors of cerebral palsy in very preterm infants: the EPIPAGE prospective population-based cohort study. Developmental Medicine and Child Neurology. 2010;52(6):e119–e125. doi: 10.1111/j.1469-8749.2010.03612.x. 20163431 [DOI] [PubMed] [Google Scholar]
- Bender G.J., Cashore W.J., Oh W. Ontogeny of bilirubin-binding capacity and the effect of clinical status in premature infants born at less than 1300 grams. Pediatrics. 2007;120(5):1067–1073. doi: 10.1542/peds.2006-3024. 17974745 [DOI] [PubMed] [Google Scholar]
- Benini R., Dagenais L., Shevell M.I. Normal imaging in patients with cerebral palsy: what does it tell us? The Journal of Pediatrics. 2013;162(2):369–374. doi: 10.1016/j.jpeds.2012.07.044. 22944004 [DOI] [PubMed] [Google Scholar]
- Bhutta A.T. Cognitive and behavioral outcomes of school-aged children who were born preterm: a meta-analysis. JAMA: the Journal of the American Medical Association. 2002;288(6):728–737. doi: 10.1001/jama.288.6.728. 12169077 [DOI] [PubMed] [Google Scholar]
- Boardman J.P. Abnormal deep grey matter development following preterm birth detected using deformation-based morphometry. Neuroimage. 2006;32(1):70–78. doi: 10.1016/j.neuroimage.2006.03.029. 16675269 [DOI] [PubMed] [Google Scholar]
- Boonkasidecha S. An optimal cut-off point of serum C-reactive protein in prediction of neonatal sepsis. Journal of the Medical Association of Thailand = Chotmaihet Thangphaet. 2013;96(Suppl. 1):S65–S70. 23724458 [PubMed] [Google Scholar]
- Brody B.A. Sequence of central nervous system myelination in human infancy. I. An autopsy study of myelination. Journal of Neuropathology and Experimental Neurology. 1987;46(3):283–301. doi: 10.1097/00005072-198705000-00005. 3559630 [DOI] [PubMed] [Google Scholar]
- De Bruïne F.T. Tractography of white-matter tracts in very preterm infants: a 2-year follow-up study. Developmental Medicine and Child Neurology. 2013;55(5):427–433. doi: 10.1111/dmcn.12099. 23441853 [DOI] [PubMed] [Google Scholar]
- Calligaris S.D. Cytotoxicity is predicted by unbound and not total bilirubin concentration. Pediatric Research. 2007;62(5):576–580. doi: 10.1203/PDR.0b013e3181568c94. 18049372 [DOI] [PubMed] [Google Scholar]
- Carlo W.A. Journal of Pediatrics. 2011;159(6):919–925. doi: 10.1016/j.jpeds.2011.05.042. 21798559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC . Metropolitan Atlanta Developmental Disabilities Surveillance Program (MADDSP) Case Definitions. National Center on Birth Defects and Developmental Disabilities, Centers for Disease Control and Prevention; Atlanta, GA.: 2013. [Google Scholar]
- Chen Y. Longitudinal regression analysis of spatial–temporal growth patterns of geometrical diffusion measures in early postnatal brain development with diffusion tensor imaging. Neuroimage. 2011;58(4):993–1005. doi: 10.1016/j.neuroimage.2011.07.006. 21784163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantinou J.C. Neurobehavioral assessment predicts differential outcome between VLBW and ELBW preterm infants. Journal of Perinatology: Official Journal of the California Perinatal Association. 2005;25(12):788–793. doi: 10.1038/sj.jp.7211403. 16292337 [DOI] [PubMed] [Google Scholar]
- Counsell S.J. Specific relations between neurodevelopmental abilities and white matter microstructure in children born preterm. Brain: a Journal of Neurology. 2008;131(12):3201–3208. doi: 10.1093/brain/awn268. 18952670 [DOI] [PubMed] [Google Scholar]
- Cowan F. Origin and timing of brain lesions in term infants with neonatal encephalopathy. Lancet. 2003;361(9359):736–742. doi: 10.1016/S0140-6736(03)12658-X. 12620738 [DOI] [PubMed] [Google Scholar]
- Delobel-Ayoub M. Behavioral problems and cognitive performance at 5 years of age after very preterm birth: the EPIPAGE Study. Pediatrics. 2009;123(6):1485–1492. doi: 10.1542/peds.2008-1216. 19482758 [DOI] [PubMed] [Google Scholar]
- Dubois J. Asynchrony of the early maturation of white matter bundles in healthy infants: quantitative landmarks revealed noninvasively by diffusion tensor imaging. Human Brain Mapping. 2008;29(1):14–27. doi: 10.1002/hbm.20363. 17318834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois J. The early development of brain white matter: a review of imaging studies in fetuses, newborns and infants. Neuroscience. 2013 doi: 10.1016/j.neuroscience.2013.12.044. 24378955 [DOI] [PubMed] [Google Scholar]
- Fan Y., Yu J.-L. Umbilical blood biomarkers for predicting early-onset neonatal sepsis. World Journal of Pediatrics: WJP. 2012;8(2):101–108. doi: 10.1007/s12519-012-0347-3. [DOI] [PubMed] [Google Scholar]
- Hack M. Young adult outcomes of very-low-birth-weight children. Seminars in Fetal & Neonatal Medicine. 2006;11(2):127–137. doi: 10.1016/j.siny.2005.11.007. 16364703 [DOI] [PubMed] [Google Scholar]
- Hays S.P., Smith E.O., Sunehag A.L. Hyperglycemia is a risk factor for early death and morbidity in extremely low birth-weight infants. Pediatrics. 2006;118(5):1811–1818. doi: 10.1542/peds.2006-0628. 17079549 [DOI] [PubMed] [Google Scholar]
- Hentges C.R. Association of late-onset neonatal sepsis with late neurodevelopment in the first two years of life of preterm infants with very low birth weight. Jornal de Pediatria. 2013;90(1):50–57. doi: 10.1016/j.jped.2013.10.002. 24148798 [DOI] [PubMed] [Google Scholar]
- Hintz S.R. Is phototherapy exposure associated with better or worse outcomes in 501- to 1000-g-birth-weight infants? Acta Paediatrica (Oslo, Norway: 1992) 2011;100(7):960–965. doi: 10.1111/j.1651-2227.2011.02175.x. 21272067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hintz S.R. Neurodevelopmental and growth outcomes of extremely low birth weight infants after necrotizing enterocolitis. Pediatrics. 2005;115(3):696–703. doi: 10.1542/peds.2004-0569. 15741374 [DOI] [PubMed] [Google Scholar]
- Hüppi P.S. Microstructural development of human newborn cerebral white matter assessed in vivo by diffusion tensor magnetic resonance imaging. Pediatric Research. 1998;44(4):584–590. doi: 10.1203/00006450-199810000-00019. 9773850 [DOI] [PubMed] [Google Scholar]
- Hüppi P.S., Dubois J. Diffusion tensor imaging of brain development. Seminars in Fetal & Neonatal Medicine. 2006;11(6):489–497. doi: 10.1016/j.siny.2006.07.006. [DOI] [PubMed] [Google Scholar]
- Inder T.E. Abnormal cerebral structure is present at term in premature infants. Pediatrics. 2005;115(2):286–294. doi: 10.1542/peds.2004-0326. 15687434 [DOI] [PubMed] [Google Scholar]
- Iwata S. Qualitative brain MRI at term and cognitive outcomes at 9 years after very preterm birth. Pediatrics. 2012;129(5):e1138–e1147. doi: 10.1542/peds.2011-1735. 22529280 [DOI] [PubMed] [Google Scholar]
- Jiang H. DtiStudio: resource program for diffusion tensor computation and fiber bundle tracking. Computer Methods and Programs in Biomedicine. 2006;81(2):106–116. doi: 10.1016/j.cmpb.2005.08.004. 16413083 [DOI] [PubMed] [Google Scholar]
- Johnson S. Academic attainment and special educational needs in extremely preterm children at 11 years of age: the EPICure study. Archives of Disease in Childhood. Fetal and Neonatal Edition. 2009;94(4):F283–F289. doi: 10.1136/adc.2008.152793. 19282336 [DOI] [PubMed] [Google Scholar]
- Johnston M.V., Hoon A.H. Possible mechanisms in infants for selective basal ganglia damage from asphyxia, kernicterus, or mitochondrial encephalopathies. Journal of Child Neurology. 2000;15(9):588–591. doi: 10.1177/088307380001500904. 11019789 [DOI] [PubMed] [Google Scholar]
- Kao L.S. Hyperglycemia and morbidity and mortality in extremely low birth weight infants. Journal of Perinatology: Official Journal of the California Perinatal Association. 2006;26(12):730–736. doi: 10.1038/sj.jp.7211593. 16929344 [DOI] [PubMed] [Google Scholar]
- Karagianni P. Neuromotor outcomes in infants with bronchopulmonary dysplasia. Pediatric Neurology. 2011;44(1):40–46. doi: 10.1016/j.pediatrneurol.2010.07.008. 21147386 [DOI] [PubMed] [Google Scholar]
- Kidokoro H. High signal intensity on T2-weighted MR imaging at term-equivalent age in preterm infants does not predict 2-year neurodevelopmental outcomes. A.J.N.R.. American Journal of Neuroradiology. 2011;32(11):2005–2010. doi: 10.3174/ajnr.A2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinney H.C. Sequence of central nervous system myelination in human infancy. II. Patterns of myelination in autopsied infants. Journal of Neuropathology and Experimental Neurology. 1988;47(3):217–234. doi: 10.1097/00005072-198805000-00003. 3367155 [DOI] [PubMed] [Google Scholar]
- Kostović I., Judas M. The development of the subplate and thalamocortical connections in the human foetal brain. Acta Paediatrica (Oslo, Norway) 1992. 2010;99(8):1119–1127. doi: 10.1111/j.1651-2227.2010.01811.x. [DOI] [PubMed] [Google Scholar]
- Larroque B. Neurodevelopmental disabilities and special care of 5-year-old children born before 33 weeks of gestation (the EPIPAGE study): a longitudinal cohort study. Lancet. 2008;371(9615):813–820. doi: 10.1016/S0140-6736(08)60380-3. 18328928 [DOI] [PubMed] [Google Scholar]
- Lee A.Y. Radiologic differences in white matter maturation between preterm and full-term infants: TBSS study. Pediatric Radiology. 2013;43(5):612–619. doi: 10.1007/s00247-012-2545-5. 23149651 [DOI] [PubMed] [Google Scholar]
- Lie K.K., Gr⊘holt E.-K., Eskild A. Association of cerebral palsy with Apgar score in low and normal birthweight infants: population based cohort study. BMJ (Clinical Research Ed.) 2010;341:c4990. doi: 10.1136/bmj.c4990. 20929920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litt J. Learning disabilities in children with very low birthweight: prevalence, neuropsychological correlates, and educational interventions. Journal of Learning Disabilities. 2005;38(2):130–141. doi: 10.1177/00222194050380020301. 15813595 [DOI] [PubMed] [Google Scholar]
- Liu X.-B. Vulnerability of premyelinating oligodendrocytes to white-matter damage in neonatal brain injury. Neuroscience bulletin. 2013;29(2):229–238. doi: 10.1007/s12264-013-1311-5. 23456565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe H.J. STRIDE—an integrated standards-based translational research informatics platform. AMIA ... Annual Symposium Proceedings [electronic resource] / AMIA Symposium. 2009;2009:391–395. 20351886 [PMC free article] [PubMed] [Google Scholar]
- Meem M. Biomarkers for diagnosis of neonatal infections: a systematic analysis of their potential as a point-of-care diagnostics. Journal of Global Health. 2011;1(2):201–209. 23198119 [PMC free article] [PubMed] [Google Scholar]
- Miller S.P., Ferriero D.M. From selective vulnerability to connectivity: insights from newborn brain imaging. Trends in Neurosciences. 2009;32(9):496–505. doi: 10.1016/j.tins.2009.05.010. 19712981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitha A. Neonatal infection and 5-year neurodevelopmental outcome of very preterm infants. Pediatrics. 2013;132(2):e372–e380. doi: 10.1542/peds.2012-3979. 23878051 [DOI] [PubMed] [Google Scholar]
- Moore T. Neurological and developmental outcome in extremely preterm children born in England in 1995 and 2006: the EPICure studies. BMJ (Clinical research ed.) 2012;345:e7961. doi: 10.1136/bmj.e7961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris B.H. Aggressive vs. conservative phototherapy for infants with extremely low birth weight. New England Journal of Medicine. 2008;359(18):1885–1896. doi: 10.1056/NEJMoa0803024. 18971491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morsing E. Cognitive function after intrauterine growth restriction and very preterm birth. Pediatrics. 2011;127(4):e874–e882. doi: 10.1542/peds.2010-1821. 21382944 [DOI] [PubMed] [Google Scholar]
- Movsas T.Z. Autism spectrum disorder is associated with ventricular enlargement in a low birth weight population. The Journal of Pediatrics. 2013;163(1):73–78. doi: 10.1016/j.jpeds.2012.12.084. 23410601 [Accessed February 25, 2013] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neil J. Diffusion tensor imaging of normal and injured developing human brain — a technical review. NMR in Biomedicine. 2002;15(7–8):543–552. doi: 10.1002/nbm.784. 12489100 [DOI] [PubMed] [Google Scholar]
- Nossin-Manor R. Deep gray matter maturation in very preterm neonates: regional variations and pathology-related age-dependent changes in magnetization transfer ratio. Radiology. 2012;263(2):510–517. doi: 10.1148/radiol.12110367. 22416249 [DOI] [PubMed] [Google Scholar]
- Nossin-Manor R. Quantitative MRI in the very preterm brain: assessing tissue organization and myelination using magnetization transfer, diffusion tensor and T? imaging. Neuroimage. 2013;64:505–516. doi: 10.1016/j.neuroimage.2012.08.086. 22982360 [DOI] [PubMed] [Google Scholar]
- Odding E., Roebroeck M.E., Stam H.J. The epidemiology of cerebral palsy: incidence, impairments and risk factors. Disability and Rehabilitation. 2006;28(4):183–191. doi: 10.1080/09638280500158422. 16467053 [DOI] [PubMed] [Google Scholar]
- Oh W. Association between peak serum bilirubin and neurodevelopmental outcomes in extremely low birth weight infants. Pediatrics. 2003;112(4):773–779. doi: 10.1542/peds.112.4.773. 14523165 [DOI] [PubMed] [Google Scholar]
- Oh W. Influence of clinical status on the association between plasma total and unbound bilirubin and death or adverse neurodevelopmental outcomes in extremely low birth weight infants. Acta Paediatrica. 2010;99(5):673–678. doi: 10.1111/j.1651-2227.2010.01688.x. 20105142 [Accessed March 12, 2013] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oishi K. Multi-contrast human neonatal brain atlas: application to normal neonate development analysis. Neuroimage. 2011;56(1):8–20. doi: 10.1016/j.neuroimage.2011.01.051. 21276861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oishi K. Quantitative evaluation of brain development using anatomical MRI and diffusion tensor imaging. International Journal of Developmental Neuroscience: the Official Journal of the International Society for Developmental Neuroscience. 2013;31(7):512–524. doi: 10.1016/j.ijdevneu.2013.06.004. 23796902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierpaoli C. Diffusion tensor MR imaging of the human brain. Radiology. 1996;201(3):637–648. doi: 10.1148/radiology.201.3.8939209. 8939209 [DOI] [PubMed] [Google Scholar]
- Pritchard V.E. Early school-based learning difficulties in children born very preterm. Early Human Development. 2009;85(4):215–224. doi: 10.1016/j.earlhumdev.2008.10.004. 19022593 [DOI] [PubMed] [Google Scholar]
- Provenzale J.M., Isaacson J., Chen S. Progression of corpus callosum diffusion-tensor imaging values during a period of signal changes consistent with myelination. AJR. American Journal of Roentgenology. 2012;198(6):1403–1408. doi: 10.2214/AJR.11.7849. 22623555 [DOI] [PubMed] [Google Scholar]
- Ramel S.E. Neonatal hyperglycemia and diminished long-term growth in very low birth weight preterm infants. Journal of Perinatology: Official Journal of the California Perinatal Association. 2013;33(11):882–886. doi: 10.1038/jp.2013.77. 23846492 [DOI] [PubMed] [Google Scholar]
- Ritter B.C. Cognitive and behavioral aspects of executive functions in children born very preterm. Child neuropsychology: A Journal on Normal and Abnormal Development in Childhood and Adolescence. 2014;20(2):129–144. doi: 10.1080/09297049.2013.773968. 23458400 [DOI] [PubMed] [Google Scholar]
- Rose J. Brain microstructural development at near-term age in very-low-birth-weight preterm infants: an atlas-based diffusion imaging study. Neuroimage. 2014;86:244–256. doi: 10.1016/j.neuroimage.2013.09.053. 24091089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose J. Neonatal brain structure on MRI and diffusion tensor imaging, sex, and neurodevelopment in very-low-birthweight preterm children. Developmental Medicine and Child Neurology. 2009;51(7):526–535. doi: 10.1111/j.1469-8749.2008.03231.x. 19459915 [DOI] [PubMed] [Google Scholar]
- Rose J. Neonatal microstructural development of the internal capsule on diffusion tensor imaging correlates with severity of gait and motor deficits. Developmental Medicine and Child Neurology. 2007;49(10):745–750. doi: 10.1111/j.1469-8749.2007.00745.x. 17880643 [DOI] [PubMed] [Google Scholar]
- Rosenbaum P. A report: the definition and classification of cerebral palsy April 2006. Developmental Medicine and Child Neurology. Supplement. 2007;109(Suppl.):8–14. 17370477 [PubMed] [Google Scholar]
- Sato Y. Is bilirubin/albumin ratio correlated with unbound bilirubin concentration? Pediatrics International: Official Journal of the Japan Pediatric Society. 2012;54(1):81–85. doi: 10.1111/j.1442-200X.2011.03457.x. 21883690 [DOI] [PubMed] [Google Scholar]
- Schlapbach L.J. Impact of sepsis on neurodevelopmental outcome in a Swiss National cohort of extremely premature infants. Pediatrics. 2011;128(2):e348–e357. doi: 10.1542/peds.2010-3338. 21768312 [DOI] [PubMed] [Google Scholar]
- Schlapbach L.J. Outcome at two years of age in a Swiss national cohort of extremely preterm infants born between 2000 and 2008. BMC Pediatrics. 2012;12:198. doi: 10.1186/1471-2431-12-198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt B. Impact of bronchopulmonary dysplasia, brain injury, and severe retinopathy on the outcome of extremely low-birth-weight infants at 18 months: results from the trial of indomethacin prophylaxis in preterms. JAMA: the Journal of the American Medical Association. 2003;289(9):1124–1129. doi: 10.1001/jama.289.9.1124. 12622582 [DOI] [PubMed] [Google Scholar]
- Song S.-K. Dysmyelination revealed through MRI as increased radial (but unchanged axial) diffusion of water. Neuroimage. 2002;17(3):1429–1436. doi: 10.1006/nimg.2002.1267. 12414282 [DOI] [PubMed] [Google Scholar]
- Sood B.G. Cytokine profiles of preterm neonates with fungal and bacterial sepsis. Pediatric Research. 2012;72(2):212–220. doi: 10.1038/pr.2012.56. 22562288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spittle A.J. Neonatal white matter abnormality predicts childhood motor impairment in very preterm children. Developmental Medicine and Child Neurology. 2011;53:1000–1006. doi: 10.1111/j.1469-8749.2011.04095.x. 22014319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan L. Quantification of deep gray matter in preterm infants at term-equivalent age using manual volumetry of 3-Tesla magnetic resonance images. Pediatrics. 2007;119(4):759–765. doi: 10.1542/peds.2006-2508. 17403847 [DOI] [PubMed] [Google Scholar]
- Stoll B.J. Late-onset sepsis in very low birth weight neonates: the experience of the NICHD Neonatal Research Network. Pediatrics. 2002;110(2 Pt 1):285–291. doi: 10.1542/peds.110.2.285. 12165580 [DOI] [PubMed] [Google Scholar]
- Thompson D.K. Corpus callosum alterations in very preterm infants: perinatal correlates and 2 year neurodevelopmental outcomes. Neuroimage. 2012;59(4):3571–3581. doi: 10.1016/j.neuroimage.2011.11.057. 22154956 [Accessed December 29, 2011] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson D.K. Hippocampal shape variations at term equivalent age in very preterm infants compared with term controls: perinatal predictors and functional significance at age 7. Neuroimage. 2013;70:278–287. doi: 10.1016/j.neuroimage.2012.12.053. 23296179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson D.K. Perinatal risk factors altering regional brain structure in the preterm infant. Brain: a Journal of Neurology. 2007;130(3):667–677. doi: 10.1093/brain/awl277. 17008333 [DOI] [PubMed] [Google Scholar]
- Tyson J.E. Does aggressive phototherapy increase mortality while decreasing profound impairment among the smallest and sickest newborns? Journal of Perinatology: Official Journal of the California Perinatal Association. 2012;32(9):677–684. doi: 10.1038/jp.2012.64. 22652561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassar R., Barnea-Goraly N., Rose J. 10th Annual World Congress of SBMT. Society for Brain Mapping and Therapeutics; Baltimore, MD: 2013. Identification of early white matter tracts in the neonatal brain: atlas-based segmentation parameters influence DTI measurements. [Google Scholar]
- Van Vliet E.O.G. Perinatal infections and neurodevelopmental outcome in very preterm and very low-birth-weight infants: a meta-analysis. JAMA Pediatrics. 2013;167(7):662–668. doi: 10.1001/jamapediatrics.2013.1199. 23689313 [DOI] [PubMed] [Google Scholar]
- Vohr B.R. Neurodevelopmental outcomes of extremely low birth weight infants <32 weeks’ gestation between 1993 and 1998. Pediatrics. 2005;116(3):635–643. doi: 10.1542/peds.2004-2247. 16143580 [DOI] [PubMed] [Google Scholar]
- Wang K. Which biomarkers reveal neonatal sepsis? PLOS ONE. 2013;8(12):e82700. doi: 10.1371/journal.pone.0082700. 24367543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watchko J.F. Kernicterus and the molecular mechanisms of bilirubin-induced CNS injury in newborns. Neuromolecular Medicine. 2006;8(4):513–530. doi: 10.1385/NMM:8:4:513. 17028373 [DOI] [PubMed] [Google Scholar]
- Williams J., Lee K.J., Anderson P.J. Prevalence of motor-skill impairment in preterm children who do not develop cerebral palsy: a systematic review. Developmental Medicine and Child Neurology. 2010;52(3):232–237. doi: 10.1111/j.1469-8749.2009.03544.x. 20002114 [DOI] [PubMed] [Google Scholar]
- Wocadlo C., Rieger I. Educational and therapeutic resource dependency at early school-age in children who were born very preterm. Early Human Development. 2006;82(1):29–37. doi: 10.1016/j.earlhumdev.2005.06.005. 16378698 [DOI] [PubMed] [Google Scholar]
- Wood N.S. The EPICure study: associations and antecedents of neurological and developmental disability at 30 months of age following extremely preterm birth. Archives of Disease in Childhood. Fetal and Neonatal Edition. 2005;90(2):F134–F140. doi: 10.1136/adc.2004.052407. 15724037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward L.J. Neonatal white matter abnormalities an important predictor of neurocognitive outcome for very preterm children. Baud, editor. PloS One. 2012;7(12):e51879. doi: 10.1371/journal.pone.0051879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward L.J. Very preterm children show impairments across multiple neurodevelopmental domains by age 4 years. Archives of Disease in Childhood. Fetal and Neonatal Edition. 2009;94(5):F339–F344. doi: 10.1136/adc.2008.146282. 19307223 [DOI] [PubMed] [Google Scholar]
- Yakovlev P.I., Lecours A. The myelogenetic cycles of regional maturation of the brain. In: Minkowski A., editor. Regional Development of the Brain in Early Life. Blackwell; Oxford: 1967. pp. 3–70. [Google Scholar]
- Yakut I. Ischemia-modified albumin may be a novel marker for the diagnosis and follow-up of necrotizing enterocolitis. Journal of Clinical Laboratory Analysis. 2014;28(3):170–177. doi: 10.1002/jcla.21661. 24395148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yerlikaya F.H. Serum ischemia-modified albumin levels at diagnosis and during treatment of late-onset neonatal sepsis. The Journal of Maternal-Fetal & Neonatal Medicine: The Official Journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies, the International Society of Perinatal Obstetricians. 2014 doi: 10.3109/14767058.2013.876621. [DOI] [PubMed] [Google Scholar]


