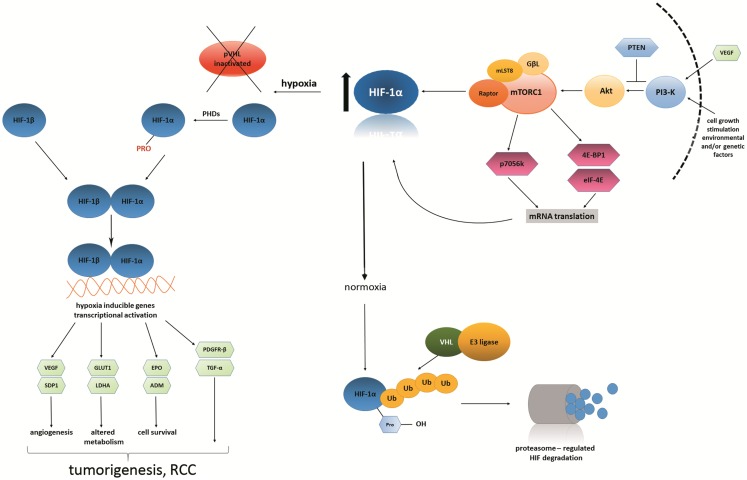
Figure 1.
Hypoxia inducible factor and VEGF link the HIF/VHL and PI3K/Akt/mTOR signaling pathways. Vascular endothelial growth factor, together with other external growth factors, activates Akt, which in turn activates mTORC1 complex The PI3K (phosphoinositide 3-kinase) pathway may be overactive because of faulty or deficient phosphatase and tensin homolog (PTEN). mTORC1 is a protein complex that functions as a specific controller of protein synthesis. It is composed of mTOR itself, a regulatory-associated mTOR protein named Raptor, mammalian lethal with SEC13 protein 8 (MLST8) and GβL, a positive regulator of the rapamycin-sensitive pathway required for the nutrient-sensitive interaction between Raptor and mTOR. What is striking is that it has been shown that mTORC1 complex, when up-regulated, subsequently up-regulates the expression of HIF-1α subunit (143–146).
Under conditions of lower oxygen tension (hypoxia), VHL tumor suppressor protein becomes inactivated, which results in constitutive activation of the HIF pathway. HIF protein is heterodimeric; it consists of two constitutively expressed subunits: β-subunit and an oxygen-sensitive α-subunit. The latter is not degraded in such conditions; therefore, it translocates to the nucleus. Inside the nucleus, it undergoes dimerization with HIF-β subunit to form transcriptionally active HIF. In consequence, HIF as a transcription factor starts to regulate many biological processes via hypoxia-inducible genes, such as: SDP1 (scan domain containing protein), GLUT1 (glucose-transporter 1), LDH (lactate dehydrogenase), EPO (erythropoietin), ADM (adrenomedullin), PDGFR-β (platelet-derived growth factor-β), TGF-α (tumor growth factor-α). Other genes activated by mTORC1 complex: p70S6 kinase, 4E-BP1 (4E-binding protein 1), eiF-4E (eukaryotic translation initiation factor 4E) (143–146).
Under conditions of normal oxygen tension (normoxia), HIF-α subunit is hydroxylated by specific prolyl-hydroxylases and subsequently targeted for rapid proteasomal degradation. This is done by the VHL tumor suppressor protein, which is active at the time. In other words, HIF protein is degraded in proteasome when prolyl-hydroxylated α-subunits are targeted to the process of ubiquitination. It occurs by high-affinity binding to the VHL E3 ubiquitin ligase. Tumorigenic processes do not occur (147, 148).