Abstract
The 12-lead electrocardiogram (ECG) is a crucial tool in the diagnosis and risk stratification of acute coronary syndrome (ACS). Unlike other 11 leads, lead aVR has been long neglected until recent years. However, recent investigations have shown that an analysis of ST-segment shift in lead aVR provides useful information on the coronary angiographic anatomy and risk stratification in ACS. ST-segment elevation in lead aVR can be caused by (1) transmural ischemia in the basal part of the interventricular septum caused by impaired coronary blood flow of the first major branch originating from the left anterior descending coronary artery; (2) transmural ischemia in the right ventricular outflow tract caused by impaired coronary blood flow of the large conal branch originating from the right coronary artery; and (3) reciprocal changes opposite to ischemic or non-ischemic ST-segment depression in the lateral limb and precordial leads. On the other hand, ST-segment depression in lead aVR can be caused by transmural ischemia in the inferolateral and apical regions. It has been recently shown that an analysis of T wave in lead aVR also provides useful prognostic information in the general population and patients with prior myocardial infarction. Cardiologists should pay more attention to the tracing of lead aVR when interpreting the 12-lead ECG in clinical practice.
Keywords: Electrocardiography, Lead aVR, ST-segment, T wave, Acute coronary syndrome
Core tip: In this article, I will review current evidence on lead aVR in the field of acute coronary syndrome.
INTRODUCTION
Lead aVR, an augmented and unipolar limb lead, was constructed to obtain specific information from the right upper portion of the heart, including the outflow tract of the right ventricle and the basal portion of the interventricular septum. However, lead aVR has been long neglected until recent years. This is thought to be because most cardiologists have considered that the tracing of lead aVR merely reflects reciprocal information from the lateral limb and precordial leads[1]. However, in the last decade, evidence indicating the importance of lead aVR in the field of acute coronary syndrome (ACS) has been accumulating. In this article, the author will review current evidence on lead aVR in the field of ACS.
ST-SEGMENT SHIFT IN LEAD AVR
Prediction of acute left main trunk occlusion
Because the left coronary artery mostly supplies approximately 75% of the left ventricular (LV) myocardial mass, acute occlusion of the left main trunk (LMT) causes life-threatening hemodynamic deterioration and malignant arrhythmias, resulting in an adverse outcome. Therefore, a rapid diagnosis and subsequent urgent revascularization with percutaneous coronary intervention (PCI) or coronary bypass surgery is very important in acute LMT occlusion. The 12-lead electrocardiogram (ECG) is a crucial tool in the diagnosis of ACS. Yamaji et al[2] compared electrocardiographic findings among 16 patients with acute LMT occlusion, 46 patients with acute left anterior descending coronary artery (LAD) occlusion, and 24 patients with acute right coronary artery (RCA) occlusion and found that ST-segment elevation > 0.05 mV in lead aVR was more common in acute LMT occlusion (88%) compared to acute LAD occlusion (43%) and acute RCA occlusion (8%). Furthermore, the magnitude of ST-segment elevation in lead aVR greater than or equal to that of ST-segment elevation in lead V1 was found to have 81% sensitivity and 80% specificity for differentiating acute LMT occlusion from acute LAD occlusion. They considered that ST-segment in lead aVR observed in acute LMT occlusion is caused by transmural ischemia in the basal part of the interventricular septum through impaired coronary blood flow of the first major septal branch arising from the LAD and that smaller ST-segment elevation in lead V1 is due to the counterbalance of injury currents produced by transmural ischemia in both the anterior and posterior walls. The Yamaji’s criterion requires validation by further studies with a large sample size.
In acute LMT occlusion, ST-segment elevation in lead aVR can also occur as a mirror image of ST-segment depression in the lateral limb and precordial leads. For example, global subendomyocardial ischemia caused by acute LMT occlusion can produce widespread ST-segment depression, especially in the lateral precordial leads, resulting in ST-segment elevation in lead aVR. In a review article, Nikus et al[3] classify the electrocardiographic findings of acute LMT occlusion into the following patterns: (1) widespread ST-segment depression with maximal changes in lead V4-6 with inverted T waves; (2) ST-segment elevation in lead aVR; and (3) anterior (anterolateral) ST-segment elevation. Ischemia-induced conduction disturbances, including right bundle branch block, left anterior fascicular block, and intraventricular conduction disturbance, are also frequently observed in acute LMT occlusion[3]. The lack of one single uniform electrocardiographic pattern of acute LMT occlusion is thought to be greatly due to the heterogeneity of the amount and localization of the ischemic jeopardized myocardium.
In summary, the electrocardiographic findings of acute LMT occlusion do not show one single uniform electrocardiographic pattern. The classification proposed by Nikus and Eskola requires validation. Whether there is a specific electrocardiographic finding to predict a poor outcome in patients with acute LMT occlusion needs to be investigated.
ST-segment elevation in lead aVR in non-ST-segment-elevation ACS
Several studies have examined the significance of ST-segment elevation in lead aVR on the admission ECG in non ST-segment elevation ACS (NSTE-ACS)[4-8]. Barrabés et al[4] examined the association between ST-segment shift in lead aVR and in-hospital mortality in 775 patients with a first non ST-segment elevation myocardial infarction (NSTEMI) and found that the rates of in-hospital mortality were 1.3% in 525 patients without ST-segment elevation in lead aVR, 8.6% in 116 patients with 0.05 mV to 0.1 mV of ST-segment elevation in lead aVR, and 19.4% in 134 patients with ST-segment elevation ≥ 0.1 mV in lead aVR. After adjusting for clinical variables, the odds ratios (ORs) for in-hospital mortality in the last 2 groups were 4.2 (95%CI: 1.5-12.2) and 6.6 (95%CI: 2.5-17.6), respectively. In 437 patients who underwent coronary arteriography within 6 mo of the onset of symptoms, the prevalence of LMT or 3-vessel disease among the 3 groups was 22.0%, 42.6%, and 66.3%, respectively. They concluded that in NSTEMI, ST-segment elevation in lead aVR is independently associated with increased in-hospital mortality probably because of severe coronary artery disease. In a GRACE substudy, including 5064 patients with NSTE-ACS, Yan et al[5] showed that neither minor (0.05-0.1 mV) nor major (> 0.1 mV) ST-segment elevation in lead aVR was an independent predictor of in-hospital and 6-mo mortality after adjusting for other validated prognosticators in the GRACE risk model. The results are inconsistent with those of Barrabés et al[4]. In the study of Yan et al[5], the prevalence of ST-segment elevation > 0.1 mV in lead aVR was only 1.5% (n = 76), which was much lower compared to the study by Barrabés et al[4]. A small number of patients with ST-segment elevation > 0.1 mV in lead aVR might have led to the negative result. In addition, entering ST-segment deviation in other leads and ST-segment elevation in lead aVR simultaneously into the multivariate analysis might have led to the negative result because all patients with ST-segment elevation > 0.1 mV in lead aVR had ST-segment deviation in other leads. Taglieri et al[6] showed that ST-segment depression ≥ 0.05 mV in any lead plus ST-segment elevation ≥ 0.1 mV in lead aVR was independently associated with culprit LMT disease and increased in-hospital and 1-year cardiovascular deaths in 1042 patients with NSTE-ACS. In these three studies[4-6], coronary arteriography was not performed in all patients.
There are a few studies[7-9] to examine the significance of ST-segment elevation in lead aVR in NSTE-ACS patients undergoing emergent coronary arteriography. Kosuge et al[7] analyzed ECGs of 310 patients with NSTE-ACS undergoing coronary arteriography and found that ST-segment elevation ≥ 0.05 mV in lead aVR was the strongest predictor of LMT or 3-vessel disease, with 78% sensitivity and 86% specificity. In another study, Kosuge et al[8] examined the prognostic value of ST-segment elevation ≥ 0.05 mV in lead aVR in 333 patients with NSTE-ACS undergoing coronary arteriography and showed that ST-segment elevation ≥ 0.05 mV in lead aVR as well as serum troponin T level ≥ 0.1 ng/mL were independent predictors of 90-d adverse outcomes, including death, myocardial infarction (MI), or urgent revascularization. When the patients were divided into 4 groups based on ST-segment shift in lead aVR and serum troponin T levels, patients with ST-segment elevation ≥ 0.05 mV in lead aVR combined with an increased serum troponin T level had the highest rates of LMT or 3-vessel disease (62%) and 90-d adverse outcomes (47%). In another study, Kosuge et al[9] examined 572 patients with NSTE-ACS undergoing coronary arteriography and showed that ST-segment elevation ≥ 0.1 mV in lead aVR identified severe LMT or 3-vessel disease (≥ 75% stenosis of LMT and/or 3-vessel disease with ≥ 90% stenosis in ≥ 2 proximal lesions of the LAD and other major epicardial arteries), with 80% sensitivity and 93% specificity.
The current evidence suggests that in patients with NSTE-ACS, ST-segment elevation in lead aVR is associated with LMT or 3-vessel disease and increased adverse events. Considering the location of lead aVR, global subendomyocardial ischemia can produce ST-segment elevation in lead aVR. Therefore, it is reasonable that ST-segment elevation in lead aVR is associated with LMT or 3-vessel disease in NSTE-ACS.
ST-segment shift in lead aVR in anterior wall STEMI caused by LAD occlusion
A few studies[10-12] have examined the significance of ST-segment sift in lead aVR on the admission ECG in first anterior wall STEMI caused by LAD occlusion. Kosuge et al[10] analyzed ECGs of 105 patients with a first anterior wall STEMI undergoing successful reperfusion and found that 35 patients with ST-segment depression ≥ 0.05 mV in lead aVR had a larger infarct size, as estimated by peak creatine kinase levels, and a lower LV ejection fraction at predischarge compared to 23 patients with ST-segment elevation ≥ 0.05 mV in lead aVR and 47 patients without ST-segment deviation in lead aVR. They speculated that ST-segment depression in lead aVR may reflect transmural ischemia extending to the apical and inferolateral walls, thereby resulting in a large MI. However, they did not evaluate the precise coronary angiographic anatomy. Accordingly, we[11] examined the association between ST-segment shift in lead aVR and emergent coronary angiographic anatomy in 261 patients with a first anterior wall STEMI and found that ST-segment depression ≥ 0.05 mV in lead aVR was associated with distal LAD occlusion (defined as occlusion of the LAD distal to the origin of the first septal branch) and a long LAD (defined as an LAD perfusing ≥ 25% of the inferior wall) and that ST-segment elevation ≥ 0.05 mV in lead aVR was associated with proximal LAD occlusion (defined as occlusion of the LAD proximal to the origin of the first septal branch) and a not-long LAD. Interestingly, patients with proximal occlusion of the long LAD, who would suffer from a large MI, had a relatively lesser degree of ST-segment shift in lead aVR. We considered that this is due to the counterbalance of injury currents produced by transmural ischemia in both the basal part of the interventricular septum and the inferolateral and apical walls. In another study[12], we examined the association between ST-segment shift in lead aVR and left ventriculography findings at predischarge in 237 patients with a first anterior wall STEMI and found that LV ejection fraction at predischarge did not differ significantly among 85 patients with ST-segment elevation ≥ 0.05 mV in lead aVR, 106 patients without ST-segment deviation, and 46 patients with ST-segment depression ≥ 0.05 mV in lead aVR. We concluded that both ST-segment elevation and depression in lead aVR may not be associated with a large infarct size in first anterior wall STEMI.
On the basis of the results of our 2 studies[11,12], the association between ST-segment shift in lead aVR and emergent coronary angiographic anatomy in first anterior wall STEMI can be summarized as follows (Figure 1): (1) ST-segment elevation in lead aVR is more common in proximal occlusion of the not-long LAD; and (2) ST-segment depression in lead aVR is more common in distal occlusion of the long LAD. Acute LAD occlusion proximal to the origin of the first septal branch can produce ST-segment elevation in lead aVR through transmural ischemia in the basal portion of the interventricular septum, and acute occlusion of the long LAD can produce ST-segment depression in lead aVR though transmural ischemia in the inferolateral and apical regions. However, it should be noted that the following conditions that can cause ST-segment elevation in lead aVR may disturb the theory: concomitant ischemia in the non-LAD region caused by multivessel disease, LV hypertrophy with strain pattern, and some types of conduction disturbances.
Figure 1.
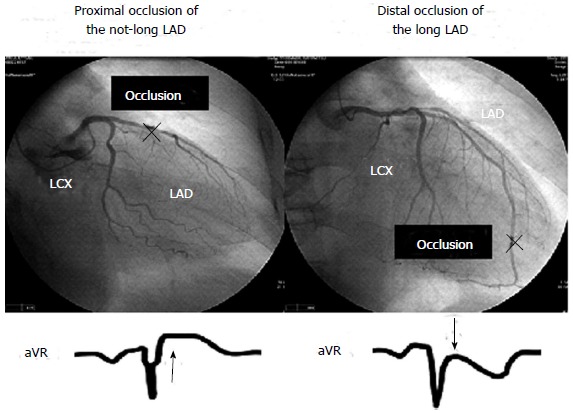
Association between ST-segment shift in aVR and coronary angiographic anatomy in a first anterior wall ST-segment elevation myocardial infarction. LAD: Left anterior descending coronary artery; LCX: left circumflex coronary artery.
The current evidence suggests that in anterior wall STEMI caused by LAD occlusion, the length of the LAD and the site of occlusion of the LAD can affect ST-segment in lead aVR. The prognostic significance of ST-segment shift in lead aVR in such STEMI needs to be clarified.
Infarct-related coronary artery and ST-segment depression in lead aVR in inferior wall STEMI
Inferior wall STEMI can be caused by RCA or left circumflex coronary artery (LCX) occlusion, although the RCA is much more likely to be the infarct-related vessel. A few studies[13-15] have examined whether ST-segment shift in lead aVR on the admission ECG can differentiate inferior wall STEMI caused by LCX occlusion from that caused by RCA occlusion. Nair et al[13] analyzed admission ECGs in 30 patients with inferior wall STEMI and found that ST-segment depression ≥ 0.1 mV in lead aVR had 80% sensitivity and 96% specificity to identify LCX occlusion. Sun et al[14] analyzed admission ECGs of 90 patients with inferior wall STEMI and showed that ST-segment depression ≥ 0.1 mV in lead aVR had 70.0% sensitivity and 94.3% specificity to identify LCX occlusion. In contrast, Kanei et al[15] showed that ST-segment depression ≥ 0.1 mV in lead aVR had a high specificity (86%) but a low sensitivity (53%) to identify LCX occlusion in 106 patients with inferior wall STEMI. Thus, the diagnostic value of ST-segment depression in lead aVR to identify LCX occlusion in inferior wall STEMI is not yet established.
The current evidence suggests that in inferior wall STEMI, ST-segment depression in lead aVR is more common in LCX occlusion than in RCA occlusion. Large population studies are needed to determine the diagnostic value of ST-segment depression in lead aVR to identify LCX occlusion in inferior wall STEMI.
Significance of ST-segment depression in lead aVR in inferior wall STEMI
A few studies[15-17] have examined the association between ST-segment depression in lead aVR on the admission ECG and infarct size in inferior wall STEMI. Menown et al[16] examined 173 patients with ST-segment elevation ≥ 0.1 mV in inferior or lateral (I, aVL, V5, and V6) leads and found that ST-segment elevation ≥ 0.1 mV in inverted lead aVR (lead -aVR) was associated with a larger infarct size, as estimated by peak creatine kinase levels. Kosuge et al[17] examined 225 patients with a first inferior wall STEMI and found that the degree of ST-segment depression in lead aVR was an independent predictor of impaired myocardial reperfusion defined as myocardial brush grade of 0 or 1. They considered that in inferior wall STEMI, ST-segment depression in lead aVR reflects transmural ischemia extending to the inferolateral and apical walls and that it therefore relates to a larger infarct size and impaired myocardial reperfusion. Kanei et al[15] reported that ST-segment depression ≥ 0.1 mV in lead aVR was associated with a large infarct size, as estimated by peak creatine kinase levels, in 86 patients with inferior wall STEMI caused by RCA occlusion but not in 19 patients with inferior wall STEMI caused by LCX occlusion. In 86 patients with RCA occlusion, the prevalence of a large posterolateral branch was higher in 12 patients with ST-segment depression ≥ 0.1 mV in lead aVR than in 74 patients without it (67% vs 16%, P = 0.0006). They considered that acute occlusion of the RCA with a large posterolateral branch occlusion can cause transmural ischemia extending to the inferolateral and apical walls, resulting in ST-segment depression in lead aVR and that it therefore relates to a larger infarct size. Since their study included only 19 patients with LCX occlusion, the association between ST-segment depression in lead aVR and infarct size in inferior wall STEMI caused by LCX occlusion needs to be further investigated.
The current evidence suggests that in inferior wall STEMI caused by RCA occlusion, ST-segment depression in lead aVR is associated with the RCA with a large posterolateral branch, which would result in a large MI. The prognostic significance of ST-segment depression in lead aVR in inferior wall STEMI needs to be determined by further studies with a large sample size.
Large population studies on the prognostic significance of ST-segment shift in lead aVR in STEMI
There are two large-population studies[18,19] to examine the prognostic significance of ST-segment shift in lead aVR on the admission ECG in STEMI. In a HERO-2 substudy, including 15315 patients with STEMI, Wong et al[18] found a U-shaped relationship between ST-segment shift in lead aVR and 30-d mortality in anterior wall STEMI. In inferior wall STEMI, only ST-segment elevation ≥ 0.1 mV in lead aVR was independently associated with increased 30-d mortality. However, the underlying mechanisms for the observations are unclear, because that study did not evaluate the coronary angiographic anatomy. In an APEX-AMI substudy[19], including 5683 patients with STEMI treated by PCI, ST-segment elevation ≥ 0.1 mV in lead aVR was independently associated with increased 90-d mortality (HR = 5.87, 95%CI: 2.09-16.5) in inferior wall STEMI, whereas ST-segment depression ≥ 0.1 mV in lead aVR was independently associated with increased 90-d mortality (HR = 1.53, 95%CI: 1.06-2.22) in non-inferior wall STEMI. However, the results have to be interpreted with some cautions. First, the precise mechanisms responsible for the observations are unclear, because that study did not evaluate the detailed coronary angiographic anatomy (the site of occlusion and the length of the coronary arteries). Second, both the inferior wall STEMI group and the non-inferior wall STEMI group included patients with STEMI caused by LMT, LAD, RCA, LCX, or graft occlusion, among whom the outcome would be different. Therefore, the heterogeneity of each group might have affected the results.
The current evidence suggests that the prognostic significance of ST-segment shift in lead aVR may differ according to the site of STEMI. The exact prognostic significance of ST-segment shift in lead aVR in anterior wall STEMI and inferior wall STEMI remains to be determined.
T-WAVE ABNORMALITY IN LEAD AVR
Although numerous studies have examined the association between T-wave abnormalities with or without ST-segment changes and cardiovascular events, the significance of T-wave abnormality in lead aVR has not been investigated until recent years. Tan et al[20] firstly examined the association between T-wave amplitude in lead aVR and cardiovascular mortality during a mean follow-up period of 4 years in 24270 male veterans whose electrocardiograms were obtained for any clinical reasons. In that study, an upright (> 0 mV) T wave in lead aVR was found to be associated with increased cardiovascular mortality after adjusting for age and heart rate (HR = 2.8, 95%CI: 2.3-3.3). Anttila et al[21] examined the prognostic impact of a positive T wave (≥ 0 mV) in lead aVR in 6254 subjects aged ≥ 30 years who participated in the field healthy examination. In that study, the positive T wave in lead aVR was observed in 2.2% of the subjects, and the relative risk for cardiovascular mortality for the positive T wave in lead aVR was 2.94 (95%CI: 1.47-2.49) after adjusting for other risk factors. In a NHANES substudy, including 7928 participants aged > 40 years, Badheka et al[22] showed that a positive (> 0 mV) T wave in lead aVR was the strongest multivariate predictor of cardiovascular mortality (OR = 3.37, 95%CI: 2.11-5.36) and that the addition of T-wave amplitude in lead aVR to the Framingham risk score improved model discrimination and calibration with better reclassification of intermediate-risk subjects. However, in these three studies[20-22], the underlying mechanisms for these observations are not identified.
A few studies[23,24] have investigated the significance of T-wave positivity in lead aVR in prior MI. We[23] examined 122 patients with anterior wall prior MI and found that 20 patients with a T wave (≥ 0.1 mV) in lead aVR had higher pulmonary arterial, pulmonary capillary wedge, and LV end-diastolic pressures, a lower cardiac index, and a lower LV ejection fraction than 102 patients without such a T wave in lead aVR. The prevalence of a long LAD was significantly higher in the former group than in the latter group (60% vs 30.4%, P = 0.01), and none of the former group had an LAD that did not reach the apex. We concluded that in anterior wall prior MI, the positive T wave in lead aVR is associated with severely reduced cardiac function, with the long LAD. In another study, we[24] examined the prognostic significance of an upright (> 0 mV) T wave in lead aVR in 167 patients with a prior MI and found that the upright T wave in lead aVR was independently associated with increased cardiac death or hospitalization for heart failure for a follow-up period of 6.5 ± 2.8 years (HR = 3.10, 95%CI: 1.23-7.82). However, because of a relatively small sample size, we could not evaluate the prognostic significance of the upright T wave in lead aVR in each of anterior wall MI and non-anterior wall MI.
The current evidence suggests that the positive T wave in lead aVR is associated with cardiovascular mortality in the general population and patients with prior MI. Further studies are needed to clarify the underlying mechanisms for increased cardiovascular mortality in subjects with a positive T wave in lead aVR in the general population and determine the prognostic significance of the positive T wave in lead aVR in anterior wall MI and non-anterior MI.
Q WAVE IN LEAD -AVR
In normal subjects, QRS configuration in lead aVR indicates QS pattern. We have noticed that a Q wave in lead -aVR (R wave in lead aVR) is sometimes observed in patients with anterior wall MI. Accordingly, we examined the association between a prominent Q wave (duration ≥ 20 ms) in lead -aVR and LV wall motion at predischarge in 87 patients with a first anterior wall STEMI[25]. In that study, 17 patients with a prominent Q wave in lead -aVR on the predischarge ECG was found to have a lower LV ejection fraction and more reduced regional wall motion in the apical and inferior regions than 70 patients without a Q wave in lead -aVR. Furthermore, the former had a higher prevalence of a long LAD compared to the latter (70.6% vs 32.9%, P = 0.01), and none of the former group had an LAD that did not reach the apex. We concluded that in anterior wall STEMI, the prominent Q wave in lead -aVR is associated with severe regional wall motion abnormality in the apical and inferior regions, with the long LAD. Further studies are needed to clarify the clinical and prognostic significance of the prominent Q wave in lead -aVR in anterior wall MI and non-anterior wall MI.
ORDERLY DISPLAY OF THE LIMB LEADS
The conventional display of the 6 precordial leads provides an anatomically contiguous view of the electrical activity progressing horizontally from the right anterior (V1) to left lateral (V6). In contrast, the conventional display of the 6 limb leads provides only a suboptimal representation of the electrical activity on the frontal plane. The 6 limb leads are anatomically better to be displayed by the following sequence: aVL, I, -aVR, II, aVF, and III (Figure 2). This orderly display of the 6 limb leads (known as the Cabrera format or sequence) provides a 150° view of the heart at regular 30° intervals. When using the orderly display, we can globally visualize the electrical activity on the frontal plane and easily understand the localization of the transmurally ischemic myocardium on the frontal plane in the setting of STEMI. The orderly display of the 6 limb leads has been routinely used in Sweden since the late 1970s. In 2009, the AHA/ACCF/HRS recommends that ECG machines should be equipped with switching systems that will allow the limb leads to be displayed and labeled appropriately in their anatomically contiguous sequences[26]. This useful display of the 6 limb leads should be routinely used in everyday clinical practice.
Figure 2.
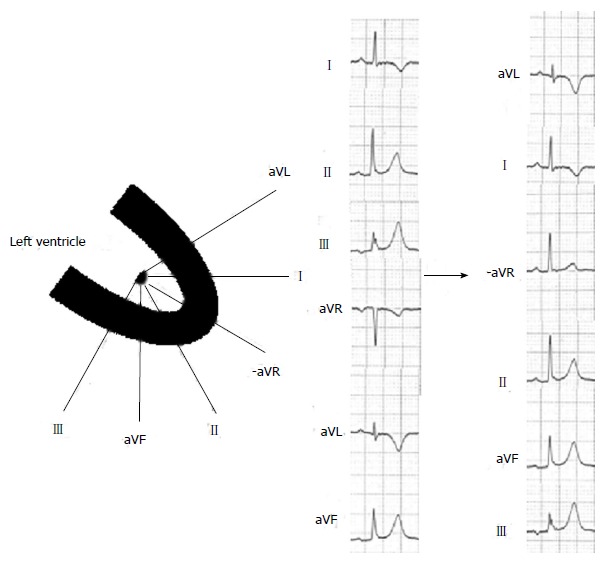
Orderly display of the 6 limb leads.
CAUTIONS WHEN INTERPTETING PREVIOUS DATA ON LEAD AVR
It should be noted that the point at which the magnitude of ST-segment elevation or depression in lead aVR was measured varies among previous studies on ST-segment shift in lead aVR. In “Third universal definition of MI”[27], abnormal ST-segment elevation or depression measured at the J point is defined. Therefore, the clinical and prognostic significance of ST-segment shift in lead aVR measured at the J point has to be determined in various conditions of ACS.
CONCLUSION
Accumulating evidence indicates that the analysis of ST-segment shift in lead aVR provides useful information on the coronary angiographic anatomy and risk stratification in various conditions of ACS. The possible mechanisms of ST-segment elevation or depression in lead aVR in ACS and the current evidence concerning the prognostic significance of ST-segment elevation or depression in lead aVR in ACS are summarized in Tables 1 and 2, respectively. It has been also shown that the analysis of T wave in lead aVR provides useful prognostic information in the general population and patients with prior MI. Cardiologists should pay more attention to ST-segment shift and T-wave positivity in lead aVR in everyday clinical practice.
Table 1.
Possible mechanisms of ST-segment elevation or depression in lead aVR and coronary angiographic anatomy in acute coronary syndrome
| Lead aVR | Possible mechanisms |
| ST-segment elevation | Global subendomyocardial ischemia caused by LMT or 3-vessel disease |
| Transmural ischemia in the basal portion of the interventricular septum caused by proximal LAD (especially, not-long LAD) occlusion | |
| Transmural ischemia in the right ventricular outflow tract caused by proximal occlusion of the RCA with a large cornal artery | |
| Reciprocal changes opposite to ischemic or non-ischemic ST-segment depression in the lateral limb and precordial leads | |
| ST-segment depression | Transmural ischemia in the inferolateral and apical regions caused by occlusion of the long LAD (especially, distal occlusion) |
| Transmural ischemia in the inferolateral and apical regions caused by occlusion of the RCA with a large posterolateral branch | |
| Transmural ischemia in the inferolateral and apical regions caused by occlusion of the LCX (especially, with impaired coronary blood flow of the obtuse marginal or posterolateral branch that perfuses the inferolateral and apical regions) |
LMT: Left main trunk; LAD: Left anterior descending coronary artery; RCA: Right coronary artery; LCX: Left circumflex coronary artery.
Table 2.
Current evidence concerning the prognostic significance of ST-segment elevation or depression in lead aVR in acute coronary syndrome
| Type of ACS | Findings of previous studies |
| NSTE-ACS | ST-segment elevation in lead aVR was independently associated with increased in-hospital mortality[4] |
| Neither minor (0.05-0.1 mV) nor major (> 0.1 mV) ST-segment elevation in lead aVR was an independent predictor of in-hospital or 6-mo mortality[5] | |
| ST-segment depression ≥ 0.05 mV in any lead plus ST-segment elevation ≥ 0.1 mV in lead aVR was independently associated with increased in-hospital and 1-year cardiovascular deaths[6] | |
| ST-segment elevation ≥ 0.05 mV in lead aVR was an independent predictor of 90-d adverse outcomes, including death, myocardial infarction, or urgent revascularization[8] | |
| Anterior wall STEMI | U-shaped relationship between ST-segment shift in lead aVR and 30-d mortality was observed[18] |
| Non-inferior wall STEMI | ST-segment depression ≥ 0.1 mV in lead aVR was independently associated with increased 90-d mortality[19] |
| Inferior wall STEMI | ST-segment elevation ≥ 0.1 mV in lead aVR was independently associated with increased 30-d mortality[18] |
| ST-segment elevation ≥ 0.1 mV in lead aVR was independently associated with increased 90-d mortality[19] |
ACS: Acute coronary syndrome; NSTE: Non ST-segment elevation; STEMI: ST-segment elevation myocardial infarction.
Footnotes
P- Reviewer: Lazzeri C, Das UN, Rassaf T S- Editor: Wen LL L- Editor: A E- Editor: Wu HL
References
- 1.Gorgels AP, Engelen DJ, Wellens HJ. Lead aVR, a mostly ignored but very valuable lead in clinical electrocardiography. J Am Coll Cardiol. 2001;38:1355–1356. doi: 10.1016/s0735-1097(01)01564-9. [DOI] [PubMed] [Google Scholar]
- 2.Yamaji H, Iwasaki K, Kusachi S, Murakami T, Hirami R, Hamamoto H, Hina K, Kita T, Sakakibara N, Tsuji T. Prediction of acute left main coronary artery obstruction by 12-lead electrocardiography. ST segment elevation in lead aVR with less ST segment elevation in lead V(1) J Am Coll Cardiol. 2001;38:1348–1354. doi: 10.1016/s0735-1097(01)01563-7. [DOI] [PubMed] [Google Scholar]
- 3.Nikus KC, Eskola MJ. Electrocardiogram patterns in acute left main coronary artery occlusion. J Electrocardiol. 2008;41:626–629. doi: 10.1016/j.jelectrocard.2008.06.020. [DOI] [PubMed] [Google Scholar]
- 4.Barrabés JA, Figueras J, Moure C, Cortadellas J, Soler-Soler J. Prognostic value of lead aVR in patients with a first non-ST-segment elevation acute myocardial infarction. Circulation. 2003;108:814–819. doi: 10.1161/01.CIR.0000084553.92734.83. [DOI] [PubMed] [Google Scholar]
- 5.Yan AT, Yan RT, Kennelly BM, Anderson FA, Budaj A, López-Sendón J, Brieger D, Allegrone J, Steg G, Goodman SG. Relationship of ST elevation in lead aVR with angiographic findings and outcome in non-ST elevation acute coronary syndromes. Am Heart J. 2007;154:71–78. doi: 10.1016/j.ahj.2007.03.037. [DOI] [PubMed] [Google Scholar]
- 6.Taglieri N, Marzocchi A, Saia F, Marrozzini C, Palmerini T, Ortolani P, Cinti L, Rosmini S, Vagnarelli F, Alessi L, et al. Short- and long-term prognostic significance of ST-segment elevation in lead aVR in patients with non-ST-segment elevation acute coronary syndrome. Am J Cardiol. 2011;108:21–28. doi: 10.1016/j.amjcard.2011.02.341. [DOI] [PubMed] [Google Scholar]
- 7.Kosuge M, Kimura K, Ishikawa T, Ebina T, Shimizu T, Hibi K, Toda N, Tahara Y, Tsukahara K, Kanna M, et al. Predictors of left main or three-vessel disease in patients who have acute coronary syndromes with non-ST-segment elevation. Am J Cardiol. 2005;95:1366–1369. doi: 10.1016/j.amjcard.2005.01.085. [DOI] [PubMed] [Google Scholar]
- 8.Kosuge M, Kimura K, Ishikawa T, Ebina T, Hibi K, Tsukahara K, Kanna M, Iwahashi N, Okuda J, Nozawa N, et al. Combined prognostic utility of ST segment in lead aVR and troponin T on admission in non-ST-segment elevation acute coronary syndromes. Am J Cardiol. 2006;97:334–339. doi: 10.1016/j.amjcard.2005.08.049. [DOI] [PubMed] [Google Scholar]
- 9.Kosuge M, Ebina T, Hibi K, Morita S, Endo M, Maejima N, Iwahashi N, Okada K, Ishikawa T, Umemura S, et al. An early and simple predictor of severe left main and/or three-vessel disease in patients with non-ST-segment elevation acute coronary syndrome. Am J Cardiol. 2011;107:495–500. doi: 10.1016/j.amjcard.2010.10.005. [DOI] [PubMed] [Google Scholar]
- 10.Kosuge M, Kimura K, Ishikawa T, Endo T, Hongo Y, Shigemasa T, Iwasawa Y, Tochikubo O, Umemura S. ST-segment depression in lead aVR predicts predischarge left ventricular dysfunction in patients with reperfused anterior acute myocardial infarction with anterolateral ST-segment elevation. Am Heart J. 2001;142:51–57. doi: 10.1067/mhj.2001.116073. [DOI] [PubMed] [Google Scholar]
- 11.Kotoku M, Tamura A, Abe Y, Kadota J. Determinants of ST-segment level in lead aVR in anterior wall acute myocardial infarction with ST-segment elevation. J Electrocardiol. 2009;42:112–117. doi: 10.1016/j.jelectrocard.2008.10.006. [DOI] [PubMed] [Google Scholar]
- 12.Goto Y, Tamura A, Kotoku M, Kadota J. ST-segment deviation in lead aVR on admission is not associated with left ventricular function at predischarge in first anterior wall ST-segment elevation acute myocardial infarction. Am J Cardiol. 2011;108:625–629. doi: 10.1016/j.amjcard.2011.04.007. [DOI] [PubMed] [Google Scholar]
- 13.Nair R, Glancy DL. ECG discrimination between right and left circumflex coronary arterial occlusion in patients with acute inferior myocardial infarction: value of old criteria and use of lead aVR. Chest. 2002;122:134–139. doi: 10.1378/chest.122.1.134. [DOI] [PubMed] [Google Scholar]
- 14.Sun TW, Wang LX, Zhang YZ. The value of ECG lead aVR in the differential diagnosis of acute inferior wall myocardial infarction. Intern Med. 2007;46:795–799. doi: 10.2169/internalmedicine.46.6411. [DOI] [PubMed] [Google Scholar]
- 15.Kanei Y, Sharma J, Diwan R, Sklash R, Vales LL, Fox JT, Schweitzer P. ST-segment depression in aVR as a predictor of culprit artery and infarct size in acute inferior wall ST-segment elevation myocardial infarction. J Electrocardiol. 2010;43:132–135. doi: 10.1016/j.jelectrocard.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 16.Menown IB, Adgey AA. Improving the ECG classification of inferior and lateral myocardial infarction by inversion of lead aVR. Heart. 2000;83:657–660. doi: 10.1136/heart.83.6.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kosuge M, Kimura K, Ishikawa T, Ebina T, Hibi K, Toda N, Umemura S. ST-segment depression in lead aVR: a useful predictor of impaired myocardial reperfusion in patients with inferior acute myocardial infarction. Chest. 2005;128:780–786. doi: 10.1378/chest.128.2.780. [DOI] [PubMed] [Google Scholar]
- 18.Wong CK, Gao W, Stewart RA, French JK, Aylward PE, White HD. The prognostic meaning of the full spectrum of aVR ST-segment changes in acute myocardial infarction. Eur Heart J. 2012;33:384–392. doi: 10.1093/eurheartj/ehr301. [DOI] [PubMed] [Google Scholar]
- 19.Alherbish A, Westerhout CM, Fu Y, White HD, Granger CB, Wagner G, Armstrong PW. The forgotten lead: does aVR ST-deviation add insight into the outcomes of ST-elevation myocardial infarction patients? Am Heart J. 2013;166:333–339. doi: 10.1016/j.ahj.2013.05.018. [DOI] [PubMed] [Google Scholar]
- 20.Tan SY, Engel G, Myers J, Sandri M, Froelicher VF. The prognostic value of T wave amplitude in lead aVR in males. Ann Noninvasive Electrocardiol. 2008;13:113–119. doi: 10.1111/j.1542-474X.2008.00210.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Anttila I, Nikus K, Nieminen T, Jula A, Salomaa V, Reunanen A, Nieminen MS, Lehtimäki T, Virtanen V, Kähönen M. Relation of positive T wave in lead aVR to risk of cardiovascular mortality. Am J Cardiol. 2011;108:1735–1740. doi: 10.1016/j.amjcard.2011.07.042. [DOI] [PubMed] [Google Scholar]
- 22.Badheka AO, Patel NJ, Grover PM, Shah N, Singh V, Deshmukh A, Mehta K, Chothani A, Hoosien M, Rathod A, et al. ST-T wave abnormality in lead aVR and reclassification of cardiovascular risk (from the National Health and Nutrition Examination Survey-III) Am J Cardiol. 2013;112:805–810. doi: 10.1016/j.amjcard.2013.04.058. [DOI] [PubMed] [Google Scholar]
- 23.Shinozaki K, Tamura A, Kadota J. Associations of positive T wave in lead aVR with hemodynamic, coronary, and left ventricular angiographic findings in anterior wall old myocardial infarction. J Cardiol. 2011;57:160–164. doi: 10.1016/j.jjcc.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 24.Torigoe K, Tamura A, Kawano Y, Shinozaki K, Kotoku M, Kadota J. Upright T waves in lead aVR are associated with cardiac death or hospitalization for heart failure in patients with a prior myocardial infarction. Heart Vessels. 2012;27:548–552. doi: 10.1007/s00380-011-0193-6. [DOI] [PubMed] [Google Scholar]
- 25.Kotoku M, Tamura A, Abe Y, Kadota J. Significance of a prominent Q wave in lead negative aVR (-aVR) in acute anterior myocardial infarction. J Electrocardiol. 2010;43:215–219. doi: 10.1016/j.jelectrocard.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 26.Wagner GS, Macfarlane P, Wellens H, Josephson M, Gorgels A, Mirvis DM, Pahlm O, Surawicz B, Kligfield P, Childers R, et al. AHA/ACCF/HRS recommendations for the standardization and interpretation of the electrocardiogram: part VI: acute ischemia/infarction: a scientific statement from the American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; the American College of Cardiology Foundation; and the Heart Rhythm Society: endorsed by the International Society for Computerized Electrocardiology. Circulation. 2009;119:e262–e270. doi: 10.1161/CIRCULATIONAHA.108.191098. [DOI] [PubMed] [Google Scholar]
- 27.Thygesen K, Alpert JS, Jaffe AS, Simoons ML, Chaitman BR, White HD, Katus HA, Lindahl B, Morrow DA, Clemmensen PM, et al. Third universal definition of myocardial infarction. Circulation. 2012;126:2020–2035. doi: 10.1161/CIR.0b013e31826e1058. [DOI] [PubMed] [Google Scholar]


