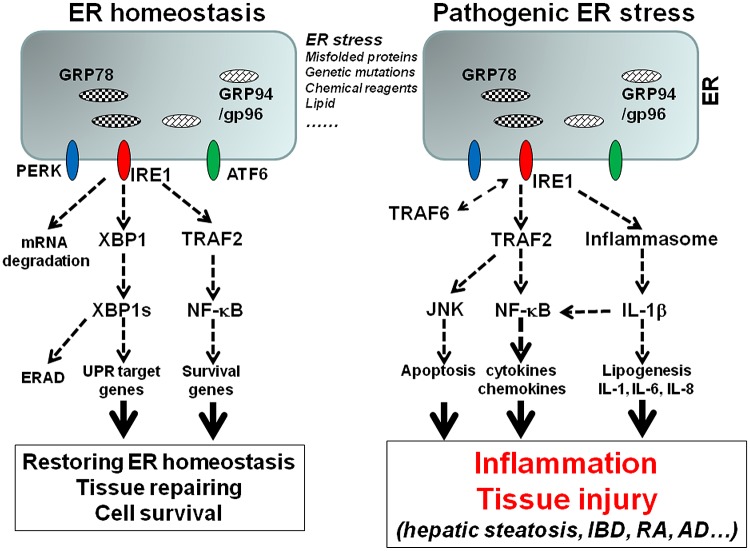
FIGURE 1.
The model of ER stress-associated inflammation. Accumulation of misfolded proteins, genetic mutations of ER stress molecules, or pharmacological compounds cause ER stress, which triggers the activation of three major UPR sensors: IRE1α, PERK, and ATF6. Activation of IRE1 pathway leads to the unconventional splicing of XBP-1, which control the transcription of a group of UPR genes including GRP78 and GRP94. The interaction of IRE1 and TRAF2 leads to NF-κB activation, which upregulates survival genes. The signaling and transcription programs initiated by IRE1 and other UPR pathways can restore the ER homeostasis. However, prolonged or unresolved ER stress leads to inflammation. Sustained interaction of IRE1 and TRAF2 leads to NF-κB and JNK activation, which promote inflammatory cytokine production and apoptosis. IRE1 is also involved in ER stress-induced inflammasome activation and IL-1β production, which in turn induces other inflammatory cytokines such as IL-6 and IL-8. In addition, TRAF6 can mediate IRE1 ubiquitination, which is required for TLR mediated optimal inflammatory cytokine production.