Abstract
The neuropeptide calcitonin gene-related peptide (CGRP) is known to play a pro-nociceptive role after peripheral nerve injury upon its release from primary afferent neurons in preclinical models of neuropathic pain. We previously demonstrated a critical role for spinal cord microglial CD40 in the development of spinal nerve L5 transection (L5Tx)-induced mechanical hypersensitivity. Herein, we investigated whether CGRP is involved in the CD40-mediated behavioral hypersensitivity. First, L5Tx was found to significantly induce CGRP expression in wild-type (WT) mice up to 14 days post-L5Tx. This increase in CGRP expression was reduced in CD40 knockout (KO) mice at day 14 post-L5Tx. Intrathecal injection of the CGRP antagonist CGRP8–37 significantly blocked L5Tx-induced mechanical hypersensitivity. In vitro, CGRP induced glial IL-6 and CCL2 production, and CD40 stimulation added to the effects of CGRP in neonatal glia. Further, there was decreased CCL2 production in CD40 KO mice compared to WT mice 21 days post-L5Tx. However, CGRP8–37 did not significantly affect spinal cord CCL2 production following L5Tx in WT mice. Altogether, these data suggest that CD40 contributes to the maintenance of behavioral hypersensitivity following peripheral nerve injury in part through two distinct pathways, the enhancement of CGRP expression and spinal cord CCL2 production.
Keywords: Spinal nerve L5 transection, glia, chemokine, cytokine, CCL2
INTRODUCTION
Chronic pain is a serious health problem that has a significant impact on modern society. Neuropathic pain, defined as pain caused by a lesion or disease of the somatosensory system (Jensen et al., 2011), is one of the most devastating kinds of chronic pain. Both microglial and astrocytic activation have been observed during the development of neuropathic pain, and decades of studies have provided ample evidence that supports the contribution of glial responses in the pathophysiology of neuropathic pain in part through the potentiation of proinflammatory responses (DeLeo et al., 2004; Tsuda et al., 2005; Milligan and Watkins, 2009). Cross-talk between central nervous system (CNS) immune responses and neuronal responses is believed to play an essential role in the development of neuropathic pain.
Calcitonin gene-related peptide (CGRP) (Arulmani et al., 2004), a 37-amino acid neuropeptide and a member of the calcitonin family of peptides, is widely distributed in both the peripheral and the central nervous systems. CGRP has both α and β forms. Although both α- and β-CGRP exhibit similar biological activity, α-CGRP is the predominant form in the sensory neurons, and as such is the focus of this current study on peripheral nerve injury-induced neuropathic pain and will be henceforth referred to as CGRP. Previous studies have demonstrated differential regulation of CGRP in spinal sensory and motor pathways after nerve injury and peripheral inflammation (Piehl et al., 1991, 1998; Weihe et al., 1995; Chen et al., 2010). However, within the nervous system, CGRP is mainly produced by medium and small diameter primary afferent neurons and is important in the modulation of pain perception. Thus, our study is concerned mostly with CGRP changes in the spinal sensory pathway. Rodent studies provide evidence of CGRP having a pro-nociceptive role in peripheral nerve injury-induced neuropathic pain. Peripheral nerve injury enhanced the evoked release of CGRP from primary afferent terminals in the spinal cord tissue, and changes in CGRP expression in the lumbar spinal cord dorsal horn were observed in various peripheral nerve injury-induced neuropathic pain models (Gardell et al., 2003; Zheng et al., 2008). Administration of a CGRP antagonist reduced tactile hypersensitivity, a behavioral sign of neuropathic pain, after both peripheral nerve injury and spinal cord injury (Bennett et al., 2000; Lee and Kim, 2007). Further, CGRP receptors have been detected on CNS glia, including astrocytes and microglia. CGRP stimulation dose-dependently increased intracellular cyclic AMP accumulation and immediate early gene expression (such as c-fos) in astrocytes and microglia (Priller et al., 1995; Reddington et al., 1995). A recent report demonstrated that CGRP treatment greatly increased inducible nitric oxide (NO) synthase expression and NO release by trigeminal ganglion glial cells in vitro (Li et al., 2008).
CD40 is a 48 kDa cell surface receptor and belongs to the tumor necrosis factor (TNF) receptor family. In the periphery, CD40 is mainly expressed by B cells, dendritic cells and macrophages, and plays critical roles in both humoral and cell-mediated immune responses (Garside et al., 1998; Grewal and Flavell, 1998; Greter et al., 2005). In the CNS, microglia, but not astrocytes and neurons, have been found to express significantly elevated levels of CD40 upon activation both in vitro and in vivo (Matyszak et al., 1999; Olson et al., 2001; Dalpke et al., 2002; Ponomarev et al., 2005; Cao et al., 2009a). CD40 stimulation has been linked to the pathogenesis of various CNS diseases including multiple sclerosis, Alzheimer’s disease, amyotrophic lateral sclerosis and human immunodeficiency virus (HIV)-1 encephalitis (Togo et al., 2000; Becher et al., 2001; Benveniste et al., 2001; D’Aversa et al., 2002; Okuno et al., 2004). Recently, we have observed a significant increase in the numbers of CD40+ microglia in the lumbar spinal cord dorsal horn postspinal nerve L5 transection (L5Tx, a murine model of neuropathic pain) and demonstrated that the CNS, particularly the spinal cord, expression of CD40 (which is almost exclusively microglial) played a critical role in the maintenance of L5Tx-induced mechanical hypersensitivity (Cao et al., 2009a). Further, microglial CD40 stimulation can lead to increased proinflammatory cytokine/chemokine production, NO release and cyclooxygenase-2 expression (Chabot et al., 1999; Tan et al., 1999a,b; Becher et al., 2000; Jana et al., 2001; D’Aversa et al., 2002; Okuno et al., 2004; Ait-Ghezala et al., 2005), and these proinflammatory responses have been implicated as the underlying mechanisms through which microglia play their roles in various neurological diseases. However, the mechanisms through which CD40 plays its role in the maintenance of L5Tx-induced neuropathic pain behavior is still unknown. It is also not clear whether there is an interaction between CGRP and CD40 in promoting the chronic pain state.
In addition, it has been well-documented that proinflammatory cytokines (such as interleukin (IL)-1β, IL-6 and TNFα) and chemokines (such as CCL2 (also known as monocyte chemoattractant protein-1)) contribute to the development of neuropathic pain (DeLeo et al., 2004; Tsuda et al., 2005; Milligan and Watkins, 2009; Gao and Ji, 2010). (Note that each known chemokine has been renamed based on the chemokine subfamilies, CC, CXC, XC or CX3C, which are defined based on the location of the cysteine residues in the amino acid sequence of the chemokines (‘C’ for cysteine).) The production of many of these proinflammatory mediators by glia has been reported (Cao et al., 2007). While earlier studies have demonstrated the roles of proinflammatory cytokines in neuropathic pain sensitization (Moalem and Tracey, 2006), more recent investigations have focused on the roles of proinflammatory chemokines. For example, CCL2 has been widely investigated in regard to its role in the development of peripheral nerve injury-induced behavioral hypersensitivity and glial activation (Abbadie et al., 2003; Zhang et al., 2007; Gao et al., 2009). CCL2 has been detected in CNS neurons, the dorsal root ganglia (DRG) and in both astrocytes and microglia (White et al., 2005; Zhang and De Koninck, 2006; Callewaere et al., 2007; Cao et al., 2007). Thus, CCL2-mediated neuronal and glial mechanisms have been suggested as pathways that promote pathological pain behaviors. However, the effects of CGRP and CD40 stimulation on glial production of CCL2, as well as all the other aforementioned proinflammatory mediators, have not been explored previously.
OBJECTIVE
In the current study, to investigate whether CGRP is involved in the CD40-mediated behavioral hypersensitivity, we first examined L5Tx-induced CGRP expression in both WT and CD40 KO mice and the effects of the CGRP antagonist CGRP8–37 on L5Tx-induced mechanical hypersensitivity. A battery of In vitro studies was conducted to test the effects of CGRP and/or CD40 stimulation on the glial production of proinflammatory mediators (IL-1β, IL-6, TNFα, CCL2, CXCL10 (also known as IP-10 (interferon gamma-induced protein 10)) and CXCL12 (also known as SDF-1 (stromal cellderived factor-1)). Then, based on the results of the In vitro studies, follow-up studies were conducted in which the CCL2 production in the lumbar spinal cords of WT, CD40 KO and CGRP antagonist-treated mice was determined.
MATERIALS AND METHODS
Animals
Male and female adult BALB/c mice were purchased from National Cancer Institute (NCI, Frederick, MD, USA) and were allowed to habituate to the institutional animal facility for at least one week before experimental use (8–9 weeks old). Breeding pairs for BALB/c CD40 KO mice were originally purchased from the Jackson Laboratory (Bar Harbor, ME, USA), bred in the animal facility of Dartmouth Medical School and then transferred to the animal facility of University of New England (UNE) (Cao et al., 2009a). Wild-type (WT) BALB/c breeding pairs were originally purchased from NCI and have been maintained at the UNE animal facility. Neonatal BALB/c mice, whose brain tissue was utilized for glial culture preparation, were obtained from these breeding pairs. All mice were group-housed with food and water ad libitum and maintained on a 12-h light/dark cycle. For all experiments that used adult mice, the ratio between male and female mice was around 1:1. Since no significant gender differences were observed for all parameters examined, data collected from male and female mice were combined and presented together in this report. The Institutional Animal Care and Use Committee at UNE approved all experimental procedures used in this study.
L5Tx and mechanical sensitivity test
WT and CD40 KO mice were randomly selected into either sham or L5Tx groups. L5Tx and sham surgeries were conducted following previously published procedures (Cao and DeLeo, 2008). Briefly, mice were anesthetized by the inhalation of isoflurane (4% for induction and 2% for maintenance) in 100% O2. To expose the L5 spinal nerve, a 1–2-cm skin incision overlying the L4-S1 section was made, and the muscle tissue was separated and retracted from the left superior articular processes and the L6 transverse process. The L6 transverse process was cleaned, and the spinal nerve L4 and L5 were exposed. The L5 spinal nerve was gently separated from the L4 spinal nerve, lifted slightly and transected with removal of a 1–2 mm segment of nerve (to prevent reconnection). The wound was irrigated with sterile saline and closed with a 6-0 silk suture for the fascia and a 3-0 polyester suture for the skin. Sham surgery was identical to the L5Tx surgery except that the L5 spinal nerve was exposed and not manipulated in any way following the partial laminectomy.
The mechanical sensitivity was measured as previously described (Cao and DeLeo, 2008). Briefly, mice were placed individually in dark plastic chambers (3′′ × 5′′ × 2′′) in non-restrained conditions. The floor of each chamber was a stainless steel mesh surface (with 1/8′′ openings). Testing chambers were elevated, so mechanical stimuli could be applied to the hind paw of each animal. During testing, each animal was subjected to stimulations from a series of von Frey filaments ranging from 0.008 to 2 g (Stoelting; Wood Dale, IL, USA) following the Up-Down paradigm (detailed in Chaplan et al., 1994). Each filament was pressed to the point of bending against the plantar surface of the ipsilateral hind paw. The 50% threshold force needed for paw withdrawal was calculated and used to represent mechanical sensitivity. For each experiment, animals were baseline tested before surgery and tested at selected times after surgery. The examiner performing the behavioral tests was blinded to the experimental groups.
Approximately the same numbers of male and female mice were used in each group. No significant gender differences were observed in all assays performed. In pilot experiments, we also confirmed that no significant differences in L5Tx-induced behavioral hypersensitivity between WT mice obtained from NCI and those bred within the UNE’s animal facility. Spinal cord tissues from treated animals were collected and analyzed via either immunohistochemistry (IHC), enzyme immunoassay (EIA) or chemokine FlowCytomix assay (see below).
IHC
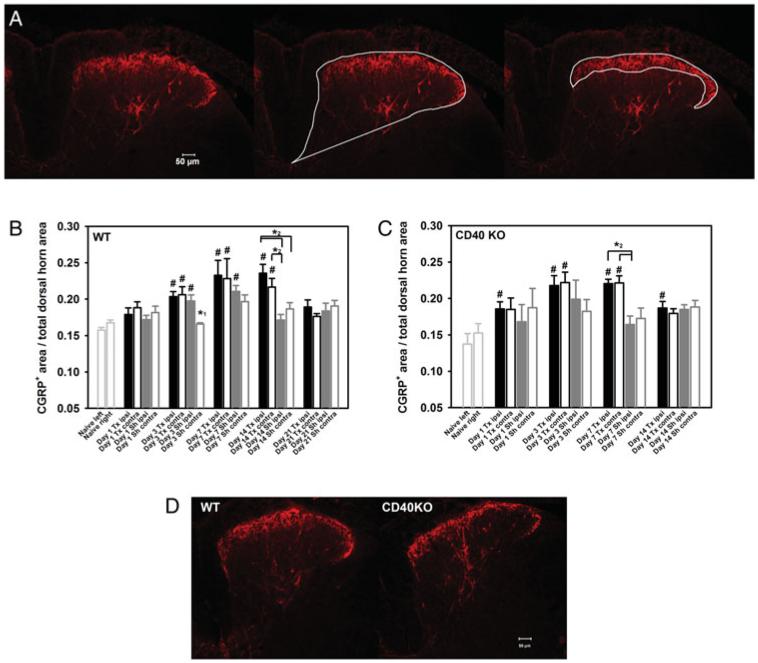
Tissue slices (15 mm) from L5 segments of lumbar spinal cord for IHC were prepared as described previously (Cao and DeLeo, 2008). Fluorescent IHC for CGRP was conducted following a modified procedure (Cao et al., 2009a). Briefly, tissue slices were first blocked with 5% normal goat serum (NGS, Jackson ImmunoResearch Laboratories, West Grove, PA, USA)/phosphate buffered saline (PBS)/0.4% Triton X-100 and then incubated with rabbit anti-CGRP (IHC6006, 1:10,000, Peninsula Laboratories, acquired by Bachem Americas, Inc., Torrance, CA, USA) overnight at 48C. After a PBS wash, slices were incubated with Cy3-anti rabbit IgG (1:800, Jackson ImmunoResearch Laboratories) for 1 h at room temperature. After a final wash, tissue slices were covered using Fluoromount-GT (VWR, Bristol, CT, USA) mounting medium. Slices were examined using a Nikon Eclipse E800 fluorescence microscope (Nikon Instruments Inc., Melville, NY, USA) with a SPOT RT Slider CCD microscope digital camera (Burlingame, CA, USA). Both ‘non-stained’ and ‘no anti-CGRP’ controls were included in each run, and no significant fluorescent signal was detected in either control. Images were analyzed using SPOT Advanced software (version 3.5.8, Diagnostic Instruments, Inc., Sterling Heights, MI, USA). To determine the CGRP expression in spinal cord dorsal horn, both the total dorsal horn area and CGRP+ area were defined and measured for each image of a L5 dorsal horn, as shown in Fig. 1A. The relative area of CGRP expression was calculated as follows: CGRP+ area/total dorsal horn area. For each sample, at least three tissue slices were analyzed and the average expression of these slices was calculated.
Fig. 1. CGRP expression in the lumbar spinal cord post-L5Tx in WT and CD40 KO mice.
CGRP IHC was performed on L5 lumbar spinal cord sections of WT and CD40 KO mice following either L5Tx or sham surgeries. The method used for analyzing CGRP expression is described in the Materials and Methods and illustrated in A. A representative image of one ipsilateral dorsal horn region is shown on the left panel. The total dorsal horn region and the CGRP+ area were defined for this image and shown in the middle and right panel, respectively. CGRP expression is represented as the ratio of CGRP+ area/total dorsal horn area. Temporal changes of CGRP expression within the L5 lumbar spinal cord following either L5Tx or sham surgeries in both WT (B, n = 3–7 per group) and CD40 KO (C, n = 3–4 per group) mice are shown. Data are presented as mean ± SEM. # indicates significant differences between the indicated group and the corresponding non-operated controls (ipsi versus left and contra versus right; ipsi = ipsilateral and contra = contralateral); * 1 indicates the significant differences between the indicated group and all other groups within the same time point; * 2 indicates the significant differences between indicated groups. Representative images of ipsilateral dorsal horn region from WT and CD40 KO mice at 14 days post-L5Tx are shown in (D). All images are at ×10 and scale bar = 50 μm.
CGRP EIA
Lumbar spinal cords (divided into ipsilateral and contralateral sides based on the surgery) were collected following transcardiac perfusion and homogenized in tissue lysis buffer (PBS supplemented with 1 mM Na2EDTA, 1% bovine serum albumin (Sigma, St. Louis, MO, USA) and a protease inhibitor cocktail (Sigma)). The supernatants were collected after centrifugation (10,000 g at 4°C for 15 min) and stored at −80°C until analyses. The protein concentration of each sample was determined with the BCA protein assay (Pierce-Thermo Scientific, Rockford, IL, USA). The CGRP EIA was performed using the CGRP EIA kit (SPI-Bio, France; cross-reactive for both rats and mice) following the manufacturer’s protocol. Following the manufacturer’s suggestion, all samples were subjected to acid extraction prior to CGRP measurement. Briefly, a mixture of sample and 2 N acetic acid (volume 1:2) was heated at 90°C for 10 min and centrifuged at 10,000 g for 10 min. The supernatants were collected, further diluted 10-fold with EIA buffer (supplied by the kit) and then CGRP was measured by EIA. All CGRP levels were normalized based on the protein concentrations of the sample.
CGRP8–37 intrathecal injection
CGRP is 37 amino acids in length. The N-terminal amino acids 1–7 are required for receptor activation and signal transduction, whereas the remainder (amino acids 8–37) of CGRP is necessary for receptor binding. Thus, a polypeptide that contains only amino acids 8–37 of CGRP (CGRP8–37) can act as a CGRP receptor antagonist (Arulmani et al., 2004). CGRP8–37 (Alpha Diagnostic, San Antonio, TX, USA) was prepared in sterile saline at 33 μg ml−1 (8.80μmM). CGRP8–37 or sterile saline were intrathecally (i.t.) delivered (5 ml per mouse) daily from day 6 to day 13 post-L5Tx in WT mice. I.t. injection was performed as described previously (Cao et al., 2009b). Briefly, under inhalation anesthesia (same as for L5Tx), a sterile 30 G needle filled with saline or CGRP8–37 was inserted into the subarachnoid space through the space between the L5 and L6 vertebral segments. A 10 μl Hamilton syringe was attached to the needle to deliver the drug into the subarachnoid space. Animals that underwent i.t. injections woke up from anesthesia a couple of minutes after the procedure, and there were no significant health issues observed in the animals that received repeated daily i.t. injections. On the days of behavioral testing, injection was performed about 30 min after the behavioral testing. Animals that underwent L5Tx but not i.t. injections were used as controls.
Primary neonatal glial cultures
Primary neonatal mixed glial cultures were prepared from 3-day-old newborn BALB/c mice following a slightly modified version of a method previously published (Hudson et al., 2008). Briefly, under aseptic conditions, whole brains were collected in sterile Hank’s Balanced Salt Solution (HBSS) (Lonza, Walkersville, MD, USA) and homogenized by the pipetting of tissue up and down with a Pasteur pipette that had a fire polished tip. To remove tissue debris, cell suspension was filtered through a 70 μm nylon cell strainer (BD Falcon, Bedford, MA, USA). Cell pellets, collected after centrifugation (281 g at 4°C for 10 min), were resuspended in complete Dulbecco’s modification of Eagle’s media (cDMEM) containing DMEM (Lonza), 10% fetal bovine serum (FBS) (PAA, New Bedford, MA, USA), 2 mM l-glutamine (Lonza), 100 IU ml−1 penicillin (Lonza), 100 μg ml−1 streptomycin (Lonza) and 100 IU ml−1 Fungizone (Gibco, Carlsbad, CA, USA). The cell suspension was distributed into 24-well culture plates (Costar, Corning, NY, USA) at one neonatal brain per plate with 500 μl of cell suspension being placed in each well. Media was replaced on days 1, 4 and 8 following the complete aspiration of old media. All cultures were maintained at 37°C in a humidified atmosphere with 5% CO2.
All cells were treated on either day 11 or 12 after the initiation of the culture, following the replacement with fresh media. For cells that did not receive lipopolysaccharide (LPS) pre-treatment, cells were treated with various concentrations of rat CGRP (no detectable endotoxin level, Alpha Diagonstics) and/or various concentrations of stimulatory anti-mouse CD40 monoclonal antibody (mAb) (clone 1C10, endotoxin level less than 0.001 ng μg−1; eBioscience, San Diego, CA, USA) simultaneously. The final concentrations of CGRP used were 0, 25, 100, 1000 and 4000 ng ml−1 (i.e. 0, 0.007, 0.026, 0.263 and 1.053 μM) and the final concentrations of anti-CD40 were 0, 0.1, 1 and 10 μg ml−1. If LPS pre-treatment was administered, Salmonella minnesota Re595 LPS (Sigma) at a final concentration of 10 ng ml−1 was given following media replacement for 1 h. Cell media were then removed, the cells were washed with HBSS and fresh media were added. Then, the aforementioned treatments of CGRP and anti-CD40 were added. For all cultures, supernatants were collected at 24 and 48 h after CGRP/anti-CD40 treatments following centrifugation of the 24-well plates (213 g at 4°C for 5 min). The supernatants were stored at −20°C until further assessment.
For all above treatments, preliminary time/dose responses were performed, and the dose ranges/times that exerted minimal cytotoxicity (determined by MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay (see below)) and produced optimal cytokine responses were chosen. The CGRP antagonist CGRP8–37 (Sigma) and anti-CD40 isotype control Ab (Rat IgG2a, eBioscience) were initially used to confirm the specificity of the CGRP and anti-CD40 treatments, respectively. Further, on the day of treatment, separate wells of non-treated cells were trypsinized, and trypan blue (Sigma) exclusion (cells were counted with a hemocytometer) was utilized to determine the numbers of viable cells per well. The cell numbers per well were consistent across experiments and averaged 1.98 ± 0.15 × 105 cells per well. In addition, the microglial percentage of these cells was analyzed via flow cytometry following a previously published procedure (Cao et al., 2007). The fluorescently labeled mAbs, APC-anti-mouse CD45 (clone 30-F11, eBioscience), FITC-anti-mouse CD11b (clone M1/70; BD Biosciences, San Diego, CA, USA) and Biotin-anti-mouse CD40 (clone 1C10, eBioscience) were used. CD45loCD11b+ cells were identified as microglia. The average microglial content of the neonatal glial cultures was 20.59 ± 2.14%. On average, CD40+ microglia made up 7.53 ± 1.98% of the microglial population in the neonatal glial cultures. As expected, CD40 expression was not observed in the non-microglial population. In the aforementioned study, we also determined that the surface CD45 and CD11b expression on mixed glial cells was not affected by the method used to harvest cells, i.e. trypsinization versus a non-enzymatic cell dissociation buffer.
Primary adult spinal cord glial cultures
The spinal cords of 8-week-old adult BALB/c mice were utilized for primary adult spinal cord mixed glial cultures following a slightly modified previously published method (Cao et al., 2007). Animals were euthanized by CO2, decapitated and entire spinal cords were collected under aseptic conditions. Following the manufacturer’s instructions, spinal cord tissue was dissociated using a papain dissociation kit (Worthington Biochemical Corporation, Lakewood, NJ, USA). The resulting cell pellets were resuspended in 20% Percoll/PBS (GE Healthcare, Piscataway, NJ, USA) and spun at 800 g, room temperature for 30 min without braking. The top layer of myelin debris was carefully removed and the cell pellets were washed twice with 1:3 (vol vol−1) cDMEM/HBSS to remove the remaining Percoll. Finally, cells were resuspended in cDMEM supplemented with 50 μM 2-mercaptoethanol (2-ME; Sigma) and distributed into 24-well plates at four mouse spinal cords per plate and 500 μl of cell suspension per well. Cells were maintained in cDMEM/2-ME, and the media were changed in the same manner described for the neonatal glial cultures at days 1, 4, 8 and 11. Cells were treated with CGRP and anti-CD40 without LPS pre-treatment as described for neonatal cultures. Supernatants were collected at 24 and 48 h after treatment. On the day of treatment, cells from non-treated wells were used to determine cell viability, and it was found that the average number of viable cells per well was 4.05 ± 0.80 × 104. Further, the percentage of microglia was examined via FACS analysis as described for the neonatal glial cultures and was found to be 7.80 ± 1.45%. On average, CD40+ microglia made up 44.88 ± 3.88% of the microglial population in the adult glial cultures. Again, CD40 expression was not observed in the non-microglial population. It should be noted that due to the differences in the two types of culture in terms of the number of total cells and microglial content, the neonatal cultures and the adult cultures actually had comparable numbers of CD40+ microglial cells.
Enzyme-linked immunosorbent assay
Mouse ELISA cytokine/chemokine detection kits were used to measure the levels of IL-1β, IL-6, TNFα, CXCL10, CXCL12 (R&D Systems, Minneapolis, MN, USA) and CCL2 (BD Biosciences) in the collected culture supernatants following the manufacturers’ protocols. The standard series were 0–1000 pg ml−1 for IL-1β, IL-6 and CCL2, 0–2000 pg ml−1 for TNFα, 0–3000 pg ml−1 for CXCL12 and 0–4000 pg ml−1 for CXCL10.
MTT assay
MTT assay (Sigma) was used to evaluate cell viability following the various treatments described above. The assay was performed following the manufacturer’s guidelines.
Griess nitrite assay
The Griess nitrite assay was used to measure the amount of nitrite in supernatants in order to estimate glial NO production. modified Griess reagent (Sigma) was used, and the assay was performed following the manufacturer’s protocol. Freshly made NaNO2 (Sigma) solutions serially diluted in DMEM served as standards (0–100 μM) for the assay (assay sensitivity is 0.4 μM).
FlowCytomix assay
Lumbar spinal cords were collected following transcardiac perfusion and homogenized in tissue lysis buffer (PBS supplemented with 1 mM Na2EDTA anda protease inhibitor cocktail (Sigma)). Supernatants were collected after centrifugation (10,000 g at 4°C for 15 min) and stored at −80°C until the FlowCytomix assay was performed. The FlowCytomix assay for CCL2 was performed with a mouse base kit and mouse chemokine bead sets (eBiosecience) following the manufacturer’s protocol using an Accuri C6 flow cytometer (Ann Arbor, MI, USA). The chemokine concentration within each sample was determined with the FlowCytomix™ Pro 2.4 software provided by the manufacturer. All chemokine levels were then normalized based on the protein concentrations of each sample as determined by the BCA protein assay (Pierce-Thermo Scientific).
Statistical analyses
All statistical analyses were performed with SigmaStat 3.5 software (Systat Software, Inc., San Jose, CA, USA). Appropriate analysis of variance (ANOVA) with treatment and time as factors were performed followed by Student–Newman–Keuls (SNK) post-hoc analysis. The Pearson correlation test was used to determine the correlation between IL-6 and CCL2 production. Correlation coefficients were compared via the Fisher z transformation. All data are presented as mean ± SEM, and P <, 0.05 was considered as statistically significant.
RESULTS
Differential lumbar spinal cord CGRP expression post-L5Tx in WT versus CD40 KO mice
Previously, we have shown that L5Tx induces mechanical hypersensitivity in BALB/c mice along with an upregulation of spinal cord micrglial CD40 expression and that this mechanical hypersensitivity is significantly reduced in CD40 KO mice after day 5 post-L5Tx. That is, CD40 contributes to the maintenance (but not the initiation) of L5Tx-induced mechanical hypersensitivity (Cao et al., 2009a). To test whether CGRP is involved in the CD40-mediated behavioral hypersensitivity, we assessed CGRP expression in the lumbar spinal cord post-L5Tx. WT BALB/c mice were subjected to either L5Tx or sham surgeries followed by CGRP IHC at days 0, 1, 3, 7, 14 and 21 post-L5Tx. Although there was no apparent change in the intensity of CGRP staining in the L5 spinal cord dorsal horn region, the area of CGRP positivity (the area containing CGRP+ nerve fibers) (described in the Materials and Methods and illustrated in Fig. 1A) gradually and significantly increased starting at day 3 after both types of surgery. In animals that received L5Tx, this increase reached a maximal level at day 7 post-L5Tx and remained elevated at day 14, but not day 21, with no significant differences detected between the ipsi- versus contralateral sides. In sham-operated mice, a different pattern was observed. In the ipsilateral side, CGRP expression also peaked at day 7 post-surgery but declined at day 14 post-L5Tx, while no significant increase was observed in the contralateral side (Fig. 1B; two-way ANOVA, Ptime < 0.001 and Pgroup = 0.009). Interestingly, when measured via EIA, total CGRP levels in the lumbar spinal cord (L4–L6) did not change significantly following either surgery (L5Tx or sham) (data not shown).
The temporal changes of CGRP expression were examined in the same manner in CD40 KO mice following either L5Tx or sham surgery. Although the changes in CGRP expression in both L5Tx- and sham-operated CD40 KO mice up to day 7 post-surgery were similar to what was observed in WT mice, no significant increases in CGRP expression in L5Tx mice were detected in both the ipsi- and contralateral sides compared to sham-operated mice at day 14 post-surgery (Fig. 1C, two-way ANOVA, Ptime < 0.001 and Pgroup = 0.069; and Fig. 1D). That is, there is a significant up-regulation of CGRP post-L5Tx at day 14 post-L5Tx in WT mice that is not observed in CD40 KO mice. These data suggest that CD40 contributes to L5Tx-induced hypersensitivity through the maintenance of the injury-induced CGRP up-regulation/release in the lumbar spinal cord.
Effects of CGRP8–37 treatment on L5Tx-induced mechanical hypersensitivity
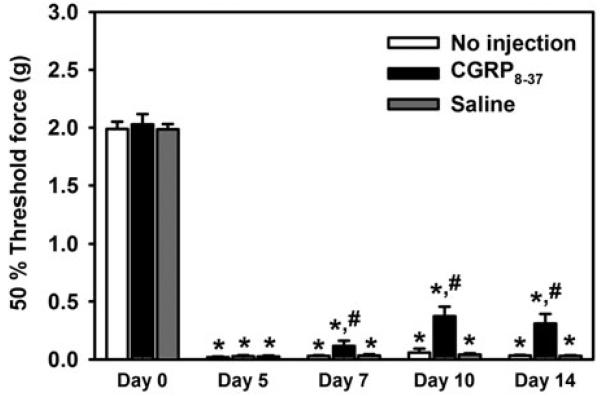
To evaluate the central role of CGRP in L5Tx-induced mechanical hypersensitivity, we i.t. injected the CGRP antagonist CGRP8–37 at a concentration of 33 μg ml−1 (8.80 mM; this dose was chosen based on a previous report; Yu et al., 1996) into WT mice daily from day 6 to day 13 post-L5Tx. Mechanical sensitivity was determined at selected time points up to day 14 post-L5Tx. It was found that the administration of CGRP8–37 significantly reduced L5Tx-induced mechanical hypersensitivity at days 7, 10 and 14 post-L5Tx (Fig. 2; two-way RM ANOVA, Ptime < 0.001, Ptreatment < 0.001 and Pinteraction < 0.001). Together with our previous studies that indicated that microglial CD40 plays a role in the maintenance of L5Tx-induced mechanical hypersensitivity (Cao et al., 2009a) and the data above that showed reduced lumbar spinal cord CGRP expression in the CD40 KO mice, the pathway: L5Tx → CD40 up-regulation → CGRP up-regulation/release → mechanical hypersensitivity was proposed and further investigated below.
Fig. 2. L5Tx-induced mechanical hypersensitivity following CGRP8–37 administration.
Adult BALB/c mice were subjected to L5Tx at day 0 followed by no injection (white), CGRP 8–37 administration (black) or saline administration (gray) from day 6 to day 13 post-L5Tx. Mechanical sensitivity was tested in all mice at selected times post-L5Tx using the up-down method. All data are presented as mean ± SEM (n = 12 per group). * indicates significant differences between the indicated group and its corresponding control group at day 0; # indicates the significant differences between the indicated group and all other groups within the same time point.
In vitro examination of the effects of CGRP and anti-CD40 on proinflammatory mediator production from mixed glia
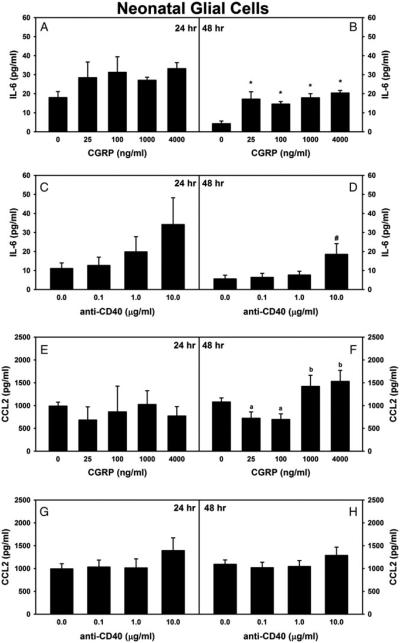
Although proinflammatory mediators can be produced from many cellular sources, it is known that the glial production of proinflammatory factors plays a pro-nociceptive role during the development of neuropathic pain (DeLeo et al., 2004; Tsuda et al., 2005; Milligan and Watkins, 2009). To test whether CGRP could directly induce the production of proinflammatory factors by glial cells (and thus contribute to L5Tx-induced mechanical hypersensitivity) and if these effects could be affected by CD40 stimulation, a series of In vitro studies that utilized mixed glia were performed. Due to the large quantity of cells that can be obtained from neonatal cultures, neonatal glia were tested initially. First, the effects of CGRP treatment or anti-CD40 (using an anti-CD40 antibody known to stimulate CD40) treatment alone were examined. The levels of the proinflammatory mediators IL-1β, TNFα, IL-6, CCL2, CXCL10, CXCL12 and NO were measured in the culture supernatants 24 and 48 h after treatment. The levels of IL-1β, TNFα and NO were too low to make accurate comparisons between treatment groups, and there were no significant changes in the levels of CXCL10 and CXCL12 following either treatment (data not shown). Both CGRP and anti-CD40 induced a trend of a dose-dependent increase in IL-6 production from mixed glial cells, and this increases reached statistical significance 48 h following either CGRP or anti-CD40 treatment (Fig. 3A–D; one-way ANOVA, in Fig. 3B, P < 0.001 and in Fig. 3D, P = 0.027). In regard to CCL2, higher doses of CGRP (1000 and 4000 ng ml−1) induced a significant increase in CCL2 secretion from mixed glial cells at 48 h after treatment (Fig. 3E,F; in Fig. 3F, one-way ANOVA, P = 0.002), whereas CD40 stimulation did not induce any statistically significant effects on glial CCL2 production (Fig. 3G,H).
Fig. 3. Effects of CGRP or anti-CD40 alone on the production of IL-6 and CCL2 from mixed neonatal glial cells.
Primary neonatal mixed glial cells were treated with various doses of either CGRP (0, 25, 100, 1000 or 4000 ng ml−1; n = 4–10 per group) or stimulatory anti-CD40 (0, 0.1, 1 or 10 μg ml−1; n = 9–13 per group) for 24 or 48 h. Supernatants were collected and analyzed for a panel of proinflammatory mediators. Data for IL-6 (A–D) and CCL2 (E–H) are presented as mean ± SEM. Within each graph, * indicates the significant differences between the indicated group and the non-treated control group; # indicates the significant differences between the indicated group and all other groups within the graph. In (F), there are significant differences between groups indicated with an ‘a’ and groups indicated with a ‘b’.
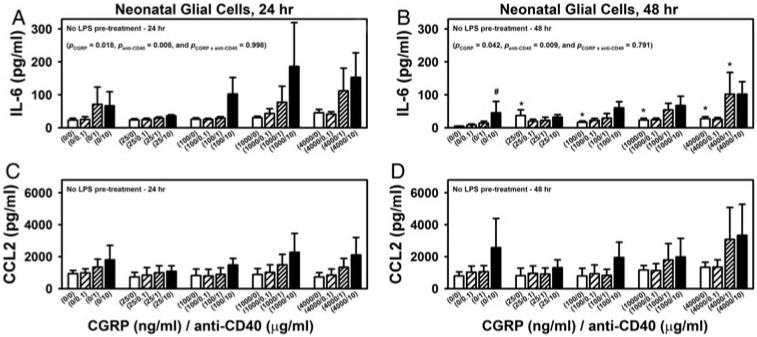
Next, to examine whether CD40 stimulation would affect CGRP’s effects on the glial production of IL-6 and CCL2, neonatal glial cells were co-treated with CGRP and anti-CD40. CGRP and CD40 co-stimulation exhibited an additive effect on IL-6 production by mixed glia at both 24 and 48 h post-treatment (Fig. 4A,B; two-way ANOVA: in Fig. 4A, PCGRP = 0.018, Panti-CD40 = 0.008 and PCGRP×anti-CD40 = 0.998, and in Fig. 4B, PCGRP = 0.042, Panti-CD40 = 0.009 and PCGRP×anti-CD40 = 0.791). Within the factor CGRP, significant differences were found between CGRP at 4000 ng ml−1 versus CGRP at 0 ng ml−1 24 h post-treatment and between every CGRP dose versus CGRP at 0 ng ml−1 48 h post-treatment. Within the factor anti-CD40, significant differences were found between anti-CD40 at 10 μg ml−1 versus anti-CD40 at 0 μg ml−1 24 h post-treatment, and anti-CD40 at 10 μg ml−1 versus all other individual anti-CD40 treated groups (including anti-CD40 at 0 μg ml−1) 48 h post-treatment. CGRP and anti-CD40 co-treatment did not induce any further significant effects on CCL2 production (Fig. 4C,D). Nevertheless, the production of CCL2 was found to be significantly correlated with the production of IL-6 regardless of the treatment at both 24 and 48 h post-treatment (P < 0.001).
Fig. 4. Effects of co-treatment of CGRP and anti-CD40 on the production of IL-6 and CCL2 from mixed neonatal glial cells.
Primary neonatal mixed glial cells were co-treated with various doses of CGRP (0, 25, 100, 1000 or 4000 ng ml−1) and stimulatory anti-CD40 (0, 0.1, 1 or 10 μg ml−1) for 24 or 48 h (A–D). Supernatants were collected and analyzed for IL-6 (A and B) and CCL2 (C and D) production via ELISA. Data are presented as mean ± SEM (n = 4 per group). P values from two-way ANOVA analyses are shown within selected graphs (details in the text). Within each graph, * indicates the significant differences between the indicated group and the corresponding control group that received 0 ng ml−1 CGRP, but the same anti-CD40 treatment; while # indicates the significant differences between the indicated group and the corresponding control group that received no anti-CD40 but the same CGRP treatment.
In a separate experiment, neonatal glial cells were pre-stimulated with a low dose of LPS (10 ng ml−1 for 1 h) prior to CGRP and anti-CD40 co-treatment. Pre-stimulation was used as it was thought that a low dose of LPS would mimic the initial inflammation and glial activation that occurs in nerve injury-induced neuropathic pain. Although the production of both IL-6 and CCL2 was dramatically enhanced in neonatal glia that received LPS pre-stimulation (~10–20-fold) compared to glial cells not pre-stimulated by LPS. No significant effects of CGRP or CD40 stimulation on IL-6 and CCL2 production were detected when cells were pre-treated with LPS (data not shown). We also determined that 10 ng ml−1 of LPS treatment for 1 h only did not induce a significant increase of IL-6 or CCL2 secretion by glial cells when compared to non-treated cells.
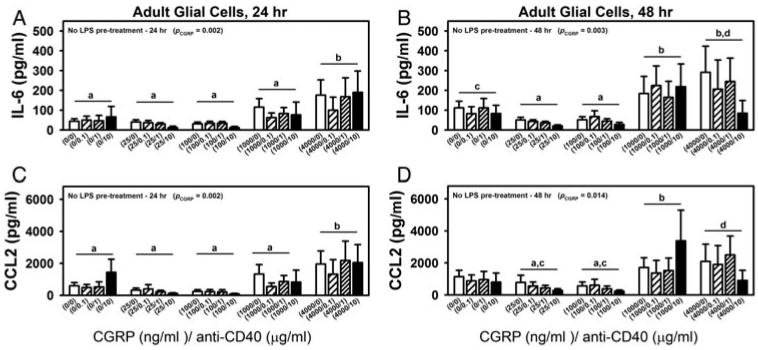
Further, to examine the effects of CGRP and CD40 stimulation in a paradigm more relevant to injury-induced neuropathic pain, spinal cord mixed glia were prepared from adult BALB/c mice. Adult spinal cord mixed glia were treated with CGRP and anti-CD40 as described for neonatal mixed glia (without LPS pre-treatment; see above). At both 24 and 48 h post-co-treatment, CGRP treatment significantly increased the production of both IL-6 and CCL2 by adult glia, but unlike what was observed with the neonatal glia, we did not observe significant effects on either IL-6 or CCL2 production at either time point following anti-CD40 treatment in adult glia (Fig. 5A–D; two-way ANOVA, in Fig. 5A, PCGRP = 0.002, in Fig. 5B, PCGRP = 0.003, in Fig. 5C, PCGRP = 0.002 and in Fig. 5D, PCGRP = 0.014). Specifically, within the factor CGRP it was found that (i) at 24 h post-treatment, significant differences were found in IL-6 and CCL2 production between CGRP at 4000 ng ml−1 versus all other treatment groups (CGRP at 0, 25, 100 or 1000 ng ml−1); and (ii) at 48 h post-treatment, IL-6 production upon CGRP stimulation was found to be significantly different at both 1000 and 4000 ng ml−1 compared to CGRP at either 25 or 100 ng ml−1. Nevertheless, similar to the findings in neonatal cultures, in adult glia, the production of CCL2 was also significantly correlated with the production of IL-6 regardless of the treatment at both 24 and 48 h post-treatment (P < 0.001). Interestingly, the association between CCL2 and IL-6 is much stronger in adult glia than in neonatal glia (Fig. 5A–D, at 24 h, r = 0.771 in neonatal glia versus r = 0.942 in adult glia; and at 48 h, r = 0.719 in neonatal glia versus r = 0.904 in adult glia; P < 0.001 for all comparisons of correlation coefficients via Fisher z transformation).
Fig. 5. Effects of co-treatment of CGRP and anti-CD40 on the production of IL-6 and CCL2 from adult spinal cord mixed glial cells.
Primary adult spinal cord mixed glial cells were co-treated with various doses of CGRP (0, 25, 100, 1000 or 4000 ng ml−1) and stimulatory anti-CD40 (0, 0.1, 1 or 10 μg ml−1) for 24 or 48 h. Supernatants were collected and analyzed for IL-6 (A and B) and CCL2 (C and D) production via ELISA. Data are presented as mean ± SEM (n = 4–6 per group). P values from two-way ANOVA analyses are shown within selected graphs (details in the text). Post hoc analyses within factor ‘CGRP’ were performed. significant differences (P < 0.05) were found between CGRP treatments that are indicated with ‘a’ versus CGRP treatments that are indicated with ‘b’. P is between 0.05 and 0.1 in the comparisons of CGRP treatments indicated with ‘c’ and those indicated with ‘d’.
Together with the in vivo data, the In vitro results, particularly those from the adult glia, suggested a possible pathway of L5Tx → CD40 upregulation → CGRP upregulation/release → glial production of IL-6 and CCL2 → mechanical hypersensitivity. However, microglial CD40 did not seem to directly affect glial IL-6 and CCL2 production, at least in adult glia.
Differential lumbar spinal cord CCL2 production post-L5Tx in WT versus CD40 KO mice
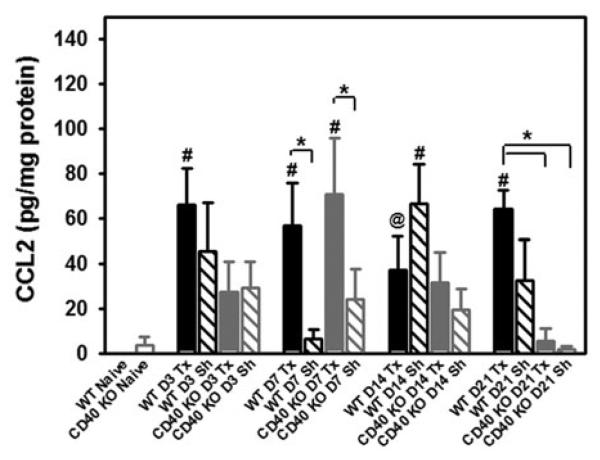
Previously, we have shown that L5Tx induced significant increases of IL-6 in the ipsilateral lumbar spinal cord in both WT and CD40 KO mice (Cao et al., 2009a), thus it is not likely that CGRP-induced IL-6 contributes significantly to the differences observed in neuropathic pain behaviors after peripheral nerve injury between WT and CD40 KO mice. It is known that CCL2 plays a critical role in nerve injury-induced behavioral hypersensitivity (Abbadie et al., 2003; Gao and Ji, 2010). Thus, CCL2 was the focus of our follow-up studies. First, to determine whether CCL2 was involved in CD40-mediated mechanical hypersensitivity following L5Tx, the levels of lumbar spinal cord CCL2 in both WT and CD40 KO mice at days 0, 1, 3, 7, 14 and 21 post-L5Tx were measured. In WT mice, L5Tx induced a significant elevation of CCL2 starting at day 1 post-L5Tx, and this elevation remained until day 21 post-L5Tx. Sham surgery only induced a temporary increase of CCL2 at day 14 post-surgery. In CD40 KO mice, L5Tx induced an increase of CCL2 that was most evident at day 7 post-L5Tx. However, unlike the result from L5Tx-operated WT mice, there was no longer a significant increase of CCL2 in CD40 KO mice subjected to L5Tx by day 21 post-surgery. Sham surgery did not induce significant increase of CCL2 in CD40 KO mice throughout the sample collection period (Fig. 6; two-way ANOVA, Ptime = 0.002, Pgroup = 0.020 and Pinteraction = 0.028).
Fig. 6. Levels of lumbar spinal cord CCL2 post-L5Tx in WT and CD40 KO mice.
Adult WT and CD40 KO BALB/c mice were subjected to L5Tx or sham surgery. At selected times post-L5Tx, lumbar spinal cords were collected and processed for the determination of tissue levels of CCL2 via FlowCytomix assay. All data are presented as mean ± SEM (n = 6 per group). # indicates significant differences between the indicated group and its corresponding naïve control group; * indicates the significant differences between the two identified groups within the same time point. P = 0.053 for the comparison between the group indicated by @ and its corresponding naïve control group.
In addition, the levels of CCL2 in the lumbar spinal cords collected from the animals treated with CGRP8–37 (same animals used in Fig. 2) were also measured. No significant changes in CCL2 levels were detected after CGRP8–37 treatment (data not shown). Thus, while CCL2 and CGRP are both CD40-dependent, they are likely independent of each other. The CD40-mediated CCL2 expression occurs at a later time point than the CD40-mediated CGRP expression. Therefore, CGRP cannot be dependent on CCL2, and based on our results, even though it can be induced by CGRP in glial culture, spinal cord CCL2 is likely not dependent on CGRP in the L5Tx model. Future studies will have to be done to determine the pathway that leads to CD40-dependent CCL2 production, and it is possible that other cellular sources of CCL2 are involved in the CD40-dependent production of CCL2. In all, the follow-up studies suggest that CD40 can mediate behavioral hypersensitivity in a CCL2-dependent manner that is independent of CGRP and a CGRP-dependent mechanism that is independent of CCL2.
CONCLUSIONS
Our results suggest that CD40 contributes to the maintenance of behavioral hypersensitivity following peripheral nerve injury through at least two independent mechanisms: (i) the enhancement of CGRP expression; and (ii) the enhancement of spinal cord CCL2 production via a mechanism that likely includes non-glial cells.
DISCUSSION
Previously, we have demonstrated that microglial CD40 contributes to the maintenance of L5Tx-induced mechanical hypersensitivity (Cao et al., 2009a). There are also pre-clinical studies that support the pro-nociceptive role of CGRP in peripheral nerve injury-induced neuropathic pain (Bennett et al., 2000; Gardell et al., 2003; Lee and Kim, 2007; Zheng et al., 2008). Here, we examined the involvement of CGRP in CD40-mediated behavioral hypersensitivity. First, the initial in vivo studies concerning CGRP expression and the effects of a CGRP antagonist on L5Tx-induced mechanical hypersensitivity indicated a possible pathway: L5Tx → CD40 upregulation → CGRP upregulation/release → mechanical hypersensitivity. To investigate whether glial-produced proinflammatory factors were potential downstream mediators of CGRP upregulation and if CD40 stimulation had direct effects on glial proinflammatory factor production, a series of in vitro studies were performed. To our knowledge, this is the first study on the direct effects of CGRP on the glial production of various proinflammatory cytokines/chemokines In vitro and how CD40 co-stimulation would affect these effects. These In vitro studies identified IL-6 and CCL2 as likely CGRP-dependent mediators. Interestingly, we did observe CD40 stimulation differentially affected CGRP-induced glial production of IL-6 and CCL2 based on the type of glia used, with additive effects found in neonatal glia and a lack of any effect in adult glia. As the microglial population is the primary cell population that expresses CD40 in the spinal cord (Hickey et al., 1992; Cao et al., 2009b and our FACS data), this difference may be attributed to the relatively lower microglial content that may lead to lower number of CD40 expressing cells following stimulation in the adult cultures compared to the neonatal cultures. (Nevertheless, before treatment, both cultures had similar numbers of CD40 expressing microglia (see the glial culture sections within Materials and Methods).) However, these data can also indicate that microglial CD40 does not have a significant direct effect on glial IL-6 and CCL2 production, particularly in adult glia (effects of CD40 on non-glial cells are possible, see below).
For the follow-up studies of the above in vivo and In vitro findings, we chose to focus on CCL2. This is due to the result from a previous study in which we observed similar patterns of L5Tx-induced lumbar spinal cord IL-6 production in WT and CD40 KO mice (Cao et al., 2009a). We examined lumbar spinal cord CCL2 production in WT and CD40 KO mice and found that the induction of CCL2 in CD40 KO mice was significantly reduced, particularly at day 21 post-L5Tx. This could be attributed to CD40-mediated actions that could be either dependent or independent of CGRP. The CGRP-dependent pathway, CD40 upregulation → CGRP release → glial CCL2 production → mechanical hypersensitivity, which was supported by the In vitro studies, was further tested by measuring the levels of lumbar spinal cord CCL2 following the treatment of the CGRP antagonist CGRP8–37. Although CGRP8–37 significantly (but not completely) reversed L5Tx-induced mechanical hypersensitivity, it did not significantly change lumbar spinal cord CCL2 production. It is possible that the dose of CGRP8–37 used was not sufficient to reverse L5Tx-induced spinal cord CCL2 production. However, despite the data from the In vitro studies, these results also suggest that in the spinal cord, CGRP-mediated production of CCL2 (from glia or non-glia) contributes less to the behavioral hypersensitivity than CCL2 production mediated by CD40 through a CGRP-independent mechanism. According to the In vitro data, particularly those from the adult glial cells, that showed CD40 did not affect glial IL-6 and CCL2 production, it is likely that a substantial amount of CCL2 involved in the latter pathway (CGRP-independent) is either produced by non-glial cells or not the direct result of the CD4O stimulation of microglia (i.e. a multi-step pathway that likely involves other cell types). Interestingly, it has been well-documented that neuronal expression of CCL2 plays a critical role in the development of neuropathic pain (White et al., 2007). Altogether, our data suggest that both a CD40-mediated, CGRP-dependent mechanism and a CD40-mediated, CCL2-dependent mechanism are involved in L5Tx-induced mechanical hypersensitivity. Further studies are necessary to delineate these pathways. Particularly, topics such as how microglial CD40 affects spinal cord CGRP expression and possibly its release from the central terminals of the primary sensory neurons, how the increased CGRP expression contributes to the maintenance of neuropathic pain behaviors, what are the other cell types involved in the CD40-dependent expression of CCL2, and how the involved cell types are affected by microglial CD40, require further investigation.
Regarding CGRP expression, unlike what has been previously reported (Zheng et al., 2008), we did not detect a significant difference in CGRP expression between the ipsilateral and contralateral sides of the spinal cord dorsal horn following L5Tx. This may be in part due to the different surgical models and rodent species used in the studies. Further, we did not observe any significant changes in total CGRP content when EIA was used, although this could be due to the sensitivity of the assays used (EIA versus IHC) and the regions analyzed (total lumbar spinal cord (L4–L6) versus L5 dorsal horn laminae I–II). In addition, it has been previously shown that CGRP expression in the spinal cord motoneurons was differentially regulated in various types of nerve injury models (including spinal nerve transection) and these changes within motoneurons may be different when compared to that within the spinal sensory pathway (Piehl et al., 1991, 1998; Zheng et al., 2008; Chen et al., 2010). Although changes in CGRP expression within the motoneurons is not our primary focus here, it can be investigated in a future study, and such an exploration may help to explain the observation mentioned above regarding the total lumbar spinal cord CGRP content following L5Tx.
The induction of glial IL-6 and CCL2 following CGRP and/or CD40 stimulation was detected with glial cells that did not receive LPS pre-treatment, but not with glia that were Briefly pre-treated with a low dose of LPS (10 ng ml−1 for 1 h only). However, this is not sufficient evidence to conclude that CGRP and CD40 exert no effect on glial IL-6 and CCL2 production when glial cells are activated. As shown in our study, glial production of both IL-6 and CCL2 are dramatically enhanced with LPS pre-treatment and this may have masked the effects of CGRP or CD40 stimulation. Further, it is known that cultured glial cells are usually in a somewhat activated state (Tawfik et al., 2006). More clinically relevant approaches for activating glia are needed to test the effects of CGRP and/or CD40 stimulation on glial responses when glial cells are activated.
Altogether, our study further supports and extends our previous observation that CD40 plays a contributing role in the maintenance phase of the development of neuropathic pain following peripheral nerve injury. This role can be in part attributed to CD40-mediated maintenance of both the upregulation of CGRP and the increase of spinal cord CCL2. However, our results indicate that it is less likely that CGRP plays its role through a CCL2-dependent mechanism. We believe further investigation of these pathways will help to identify novel drug targets for neuropathic pain treatment in the future.
ACKNOWLEDGEMENTS
The authors would like to thank Dr. Stephen Pelsue in the Southern Maine University for his help in flow cytometric analysis. This work was supported by 1K01DA023503-01A1 (PI Cao) from NIH/NIDA.
Footnotes
Statement of interest
None.
REFERENCES
- Abbadie C, Lindia JA, Cumiskey AM, Peterson LB, Mudgett JS, Bayne EK, et al. Impaired neuropathic pain responses in mice lacking the chemokine receptor CCR2. Proceedings of the National Academy of Sciences of the U.S.A. 2003;100:7947–7952. doi: 10.1073/pnas.1331358100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ait-Ghezala G, Mathura VS, Laporte V, Quadros A, Paris D, Patel N, et al. Genomic regulation after CD40 stimulation in microglia: relevance to Alzheimer’s disease. Brain Research Molecular Brain Research. 2005;140:73–85. doi: 10.1016/j.molbrainres.2005.07.014. [DOI] [PubMed] [Google Scholar]
- Arulmani U, MaassenVanDenBrink A, Villalón CM, Saxena PR. Calcitonin gene-related peptide and its role in migraine patho-physiology. European Journal of Pharmacology. 2004;500:315–330. doi: 10.1016/j.ejphar.2004.07.035. [DOI] [PubMed] [Google Scholar]
- Becher B, Blain M, Antel JP. CD40 engagement stimulates IL-12 p70 production by human microglial cells: basis for Th1 polarization in the CNS. Journal of Neuroimmunology. 2000;102:44–50. doi: 10.1016/s0165-5728(99)00152-6. [DOI] [PubMed] [Google Scholar]
- Becher B, Durell BG, Miga AV, Hickey WF, Noelle RJ. The clinical course of experimental autoimmune encephalomyelitis and inflammation is controlled by the expression of CD40 within the central nervous system. Journal of Experimental Medicine. 2001;193:967–974. doi: 10.1084/jem.193.8.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett AD, Chastain KM, Hulsebosch CE. Alleviation of mechanical and thermal allodynia by CGRP8-37 in a rodent model of chronic central pain. Pain. 2000;86:163–175. doi: 10.1016/s0304-3959(00)00242-6. [DOI] [PubMed] [Google Scholar]
- Benveniste EN, Nguyen VT, O’Keefe GM. Immunological aspects of microglia: relevance to Alzheimer’s disease. Neurochemistry International. 2001;39:381–391. doi: 10.1016/s0197-0186(01)00045-6. [DOI] [PubMed] [Google Scholar]
- Callewaere C, Banisadr G, Rostene W, Parsadaniantz SM. Chemokines and chemokine receptors in the brain: implication in neuroendocrine regulation. Journal of Molecular Endocrinology. 2007;38:355–363. doi: 10.1677/JME-06-0035. [DOI] [PubMed] [Google Scholar]
- Cao L, DeLeo JA. CNS-infiltrating CD4+ T lymphocytes contribute to murine spinal nerve transection-induced neuropathic pain. European Journal Immunology. 2008;38:448–458. doi: 10.1002/eji.200737485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao L, Fei L, Chang TT, DeLeo JA. Induction of interleukin-1beta by interleukin-4 in lipopolysaccharide-treated mixed glial cultures: microglial-dependent effects. Journal of Neurochemistry. 2007;102:408–419. doi: 10.1111/j.1471-4159.2007.04588.x. [DOI] [PubMed] [Google Scholar]
- Cao L, Palmer CD, Malon JT, De Leo JA. Critical role of microglial CD40 in the maintenance of mechanical hypersensitivity in a murine model of neuropathic pain. European Journal of Immunology. 2009a;39:3562–3569. doi: 10.1002/eji.200939657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao L, Tanga FY, Deleo JA. The contributing role of CD14 in toll-like receptor 4 dependent neuropathic pain. Neuroscience. 2009b;158:896–903. doi: 10.1016/j.neuroscience.2008.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chabot S, Williams G, Hamilton M, Sutherland G, Yong VW. Mechanisms of IL-10 production in human microglia-T cell interaction. Journal of Immunology. 1999;162:6819–6828. [PubMed] [Google Scholar]
- Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. Journal of Neuroscience Methods. 1994;53:55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- Chen LJ, Zhang FG, Li J, Song HX, Zhou LB, Yao BC, et al. Expression of calcitonin gene-related peptide in anterior and posterior horns of the spinal cord after brachial plexus injury. Journal of Clinical Neuroscience. 2010;17:87–91. doi: 10.1016/j.jocn.2009.03.042. [DOI] [PubMed] [Google Scholar]
- Dalpke AH, Schafer MK, Frey M, Zimmermann S, Tebbe J, Weihe E, et al. Immunostimulatory CpG-DNA activates murine microglia. Journal of Immunology. 2002;168:4854–4863. doi: 10.4049/jimmunol.168.10.4854. [DOI] [PubMed] [Google Scholar]
- D’Aversa TG, Weidenheim KM, Berman JW. CD40–CD40L interactions induce chemokine expression by human microglia: implications for human immunodeficiency virus encephalitis and multiple sclerosis. American Journal of Pathology. 2002;160:559–567. doi: 10.1016/S0002-9440(10)64875-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLeo JA, Tanga FY, Tawfik VL. Neuroimmune activation and neuroinflammation in chronic pain and opioid tolerance/hyperalgesia. Neuroscientist. 2004;10:40–52. doi: 10.1177/1073858403259950. [DOI] [PubMed] [Google Scholar]
- Gao YJ, Ji RR. Chemokines, neuronal-glial interactions, and central processing of neuropathic pain. Pharmacological Therapy. 2010;126:56–68. doi: 10.1016/j.pharmthera.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao YJ, Zhang L, Samad OA, Suter MR, Yasuhiko K, Xu ZZ, et al. JNK-induced MCP-1 production in spinal cord astrocytes contributes to central sensitization and neuropathic pain. Journal of Neuroscience. 2009;29:4096–4108. doi: 10.1523/JNEUROSCI.3623-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardell LR, Vanderah TW, Gardell SE, Wang R, Ossipov MH, Lai J, et al. Enhanced evoked excitatory transmitter release in experimental neuropathy requires descending facilitation. Journal of Neuroscience. 2003;23:8370–8379. doi: 10.1523/JNEUROSCI.23-23-08370.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garside P, Ingulli E, Merica RR, Johnson JG, Noelle RJ, Jenkins MK. Visualization of specific B and T lymphocyte interactions in the lymph node. Science. 1998;281:96–99. doi: 10.1126/science.281.5373.96. [DOI] [PubMed] [Google Scholar]
- Greter M, Heppner FL, Lemos MP, Odermatt BM, Goebels N, Laufer T, et al. Dendritic cells permit immune invasion of the CNS in an animal model of multiple sclerosis. Nature Medicine. 2005;11:328–334. doi: 10.1038/nm1197. [DOI] [PubMed] [Google Scholar]
- Grewal IS, Flavell RA. CD40 and CD154 in cell-mediated immunity. Annual Review of Immunology. 1998;16:111–135. doi: 10.1146/annurev.immunol.16.1.111. [DOI] [PubMed] [Google Scholar]
- Hickey WF, Vass K, Lassmann H. Bone marrow-derived elements in the central nervous system: an immunohistochemical and ultrastructural survey of rat chimeras. Journal of Neuropathology and Experimental Neurology. 1992;51:246–256. doi: 10.1097/00005072-199205000-00002. [DOI] [PubMed] [Google Scholar]
- Hudson CA, Christophi GP, Gruber RC, Wilmore JR, Lawrence DA, Massa PT. Induction of IL-33 expression and activity in central nervous system glia. Journal of Leukocyte Biology. 2008;84:631–643. doi: 10.1189/jlb.1207830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jana M, Liu X, Koka S, Ghosh S, Petro TM, Pahan K. Ligation of CD40 stimulates the induction of nitric-oxide synthase in microglial cells. Journal of Biological Chemistry. 2001;276:44527–44533. doi: 10.1074/jbc.M106771200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen TS, Baron R, Haanpaãaã M, Kalso E, Loeser JD, Rice ASC, et al. A new definition of neuropathic pain. Pain. 2011;152:2204–2205. doi: 10.1016/j.pain.2011.06.017. [DOI] [PubMed] [Google Scholar]
- Lee SE, Kim J-H. Involvement of substance P and calcitonin gene-related peptide in development and maintenance of neuropathic pain from spinal nerve injury model of rat. Neuroscience Research. 2007;58:245–249. doi: 10.1016/j.neures.2007.03.004. [DOI] [PubMed] [Google Scholar]
- Li J, Vause CV, Durham PL. Calcitonin gene-related peptide stimulation of nitric oxide synthesis and release from trigeminal ganglion glial cells. Brain Research. 2008;1196:22–32. doi: 10.1016/j.brainres.2007.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matyszak MK, Denis-Donini S, Citterio S, Longhi R, Granucci F, Ricciardi-Castagnoli P. Microglia induce myelin basic protein-specific T cell anergy or T cell activation, according to their state of activation. European Journal of Immunology. 1999;29:3063–3076. doi: 10.1002/(SICI)1521-4141(199910)29:10<3063::AID-IMMU3063>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Milligan ED, Watkins LR. Pathological and protective roles of glia in chronic pain. Nature Review of Neuroscience. 2009;10:23–36. doi: 10.1038/nrn2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moalem G, Tracey DJ. Immune and inflammatory mechanisms in neuropathic pain. Brain Research Review. 2006;51:240–264. doi: 10.1016/j.brainresrev.2005.11.004. [DOI] [PubMed] [Google Scholar]
- Okuno T, Nakatsuji Y, Kumanogoh A, Koguchi K, Moriya M, Fujimura H, et al. Induction of cyclooxygenase-2 in reactive glial cells by the CD40 pathway: relevance to amyotrophic lateral sclerosis. Journal of Neurochemistry. 2004;91:404–412. doi: 10.1111/j.1471-4159.2004.02727.x. [DOI] [PubMed] [Google Scholar]
- Olson JK, Girvin AM, Miller SD. Direct activation of innate and antigen-presenting functions of microglia following infection with Theiler’s virus. Journal of Virology. 2001;75:9780–9789. doi: 10.1128/JVI.75.20.9780-9789.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piehl F, Arvidsson U, Johnson H, Cullheim S, Villar M, Dagerlind A, et al. Calcitonin gene-related peptide (CGRP)-like immuno-reactivity and CGRP mRNA in rat spinal cord motoneurons after different types of lesions. European Journal of Neuroscience. 1991;3:737–757. doi: 10.1111/j.1460-9568.1991.tb01670.x. [DOI] [PubMed] [Google Scholar]
- Piehl F, Hammarberg H, Tabar G, Hokfelt T, Cullheim S. Changes in the mRNA expression pattern, with special reference to calcitonin gene-related peptide, after axonal injuries in rat motoneurons depends on age and type of injury. Experimental Brain Research. 1998;119:191–204. doi: 10.1007/s002210050333. [DOI] [PubMed] [Google Scholar]
- Ponomarev ED, Shriver LP, Maresz K, Dittel BN. Microglial cell activation and proliferation precedes the onset of CNS autoimmunity. Journal of Neuroscience Research. 2005;81:374–389. doi: 10.1002/jnr.20488. [DOI] [PubMed] [Google Scholar]
- Priller J, Haas CA, Reddington M, Kreutzberg GW. Calcitonin gene-related peptide and ATP induce immediate early gene expression in cultured rat microglial cells. Glia. 1995;15:447–457. doi: 10.1002/glia.440150408. [DOI] [PubMed] [Google Scholar]
- Reddington M, Priller J, Treichel J, Haas C, Kreutzberg GW. Astrocytes and microglia as potential targets for calcitonin gene related peptide in the central nervous system. Canadian Journal of Physiology and Pharmacology. 1995;73:1047–1049. doi: 10.1139/y95-148. [DOI] [PubMed] [Google Scholar]
- Tan J, Town T, Paris D, Mori T, Suo Z, Crawford F, et al. Microglial activation resulting from CD40-CD40L interaction after beta-amyloid stimulation. Science. 1999a;286:2352–2355. doi: 10.1126/science.286.5448.2352. [DOI] [PubMed] [Google Scholar]
- Tan J, Town T, Paris D, Placzek A, Parker T, Crawford F, et al. Activation of microglial cells by the CD40 pathway: relevance to multiple sclerosis. Journal of Neuroimmunology. 1999b;97:77–85. doi: 10.1016/s0165-5728(99)00053-3. [DOI] [PubMed] [Google Scholar]
- Tawfik VL, Lacroix-Fralish ML, Bercury KK, Nutile-McMenemy N, Harris BT, Deleo JA. Induction of astrocyte differentiation by propentofylline increases glutamate transporter expression In vitro: heterogeneity of the quiescent phenotype. Glia. 2006;54:193–203. doi: 10.1002/glia.20365. [DOI] [PubMed] [Google Scholar]
- Togo T, Akiyama H, Kondo H, Ikeda K, Kato M, Iseki E, et al. Expression of CD40 in the brain of Alzheimer’s disease and other neurological diseases. Brain Research. 2000;885:117–121. doi: 10.1016/s0006-8993(00)02984-x. [DOI] [PubMed] [Google Scholar]
- Tsuda M, Inoue K, Salter MW. Neuropathic pain and spinal microglia: a big problem from molecules in ‘small’ glia. Trends in Neuroscience. 2005;28:101–107. doi: 10.1016/j.tins.2004.12.002. [DOI] [PubMed] [Google Scholar]
- Weihe E, Nohr D, Schafer MK, Persson S, Ekstrom G, Kallstrom J, et al. Calcitonin gene related peptide gene expression in collagen-induced arthritis. Canadian Journal of Physiology and Pharmacology. 1995;73:1015–1019. doi: 10.1139/y95-142. [DOI] [PubMed] [Google Scholar]
- White FA, Jung H, Miller RJ. Chemokines and the patho-physiology of neuropathic pain. Proceedings of the National Academy of Sciences of the U.S.A. 2007;104:20151–20158. doi: 10.1073/pnas.0709250104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White FA, Sun J, Waters SM, Ma C, Ren D, Ripsch M, et al. Excitatory monocyte chemoattractant protein-1 signaling is up-regulated in sensory neurons after chronic compression of the dorsal root ganglion. Proceedings of the National Academy of Sciences of the U.S.A. 2005;102:14092–14097. doi: 10.1073/pnas.0503496102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu LC, Hansson P, Lundeberg T. The calcitonin gene-related peptide antagonist CGRP8-37 increases the latency to withdrawal responses bilaterally in rats with unilateral experimental mononeuropathy, an effect reversed by naloxone. Neuroscience. 1996;71:523–531. doi: 10.1016/0306-4522(95)00428-9. [DOI] [PubMed] [Google Scholar]
- Zhang J, De Koninck Y. Spatial and temporal relationship between monocyte chemoattractant protein-1 expression and spinal glial activation following peripheral nerve injury. Journal of Neurochemistry. 2006;97:772–783. doi: 10.1111/j.1471-4159.2006.03746.x. [DOI] [PubMed] [Google Scholar]
- Zhang J, Shi XQ, Echeverry S, Mogil JS, De Koninck Y, Rivest S. Expression of CCR2 in both resident and bone marrow-derived microglia plays a critical role in neuropathic pain. Journal of Neuroscience. 2007;27:12396–12406. doi: 10.1523/JNEUROSCI.3016-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng LF, Wang R, Xu YZ, Yi XN, Zhang JW, Zeng ZC. Calcitonin gene-related peptide dynamics in rat dorsal root ganglia and spinal cord following different sciatic nerve injuries. Brain Research. 2008;1187:20–32. doi: 10.1016/j.brainres.2007.10.044. [DOI] [PubMed] [Google Scholar]