Figure 5. SOX2 is Required for Peripheral Neuronal Differentiation.
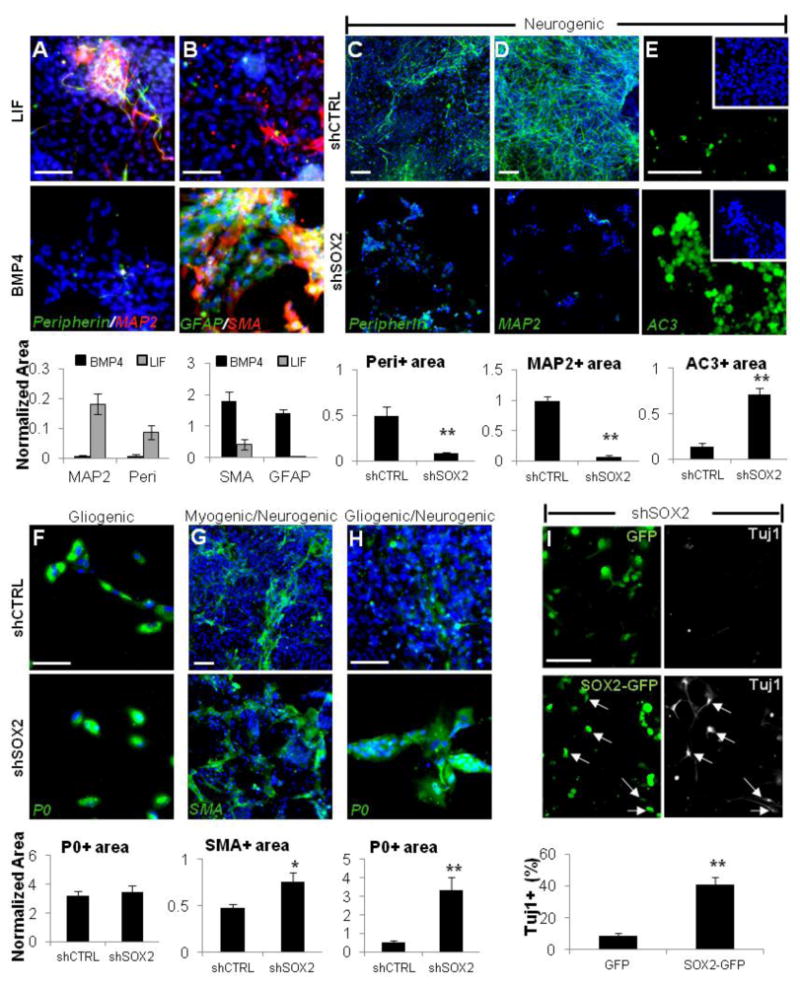
(A and B) hESC-NC cultured with LIF (upper row) are enriched for Peripherin and MAP2; the addition of BMP4 (lower row) enriches for the smooth muscle (SMA) and glial (GFAP) markers. (C and D) Under the neurogenic conditions, differentiation into Peripherin- (C) and MAP2- (D) positive neurons is abolished when SOX2 expression is downregulated using dox-inducible SOX2 shRNA, but not control scrambled shCTRL.
(E) Immunostaining for active Caspase3 (AC3) under neurogenic culture conditions in shCTRL (upper panel) and shSOX2 cells (lower panel) cultured in the presence of dox.
(F–H) Expression of SOX2 shRNA versus scrambled shCTRL under gliogenic conditions did not affect P0+ cells (F) and resulted in the increase in SMA+ cells (G) and P0+ cells (H) under the mixed conditions.
(I) SOX2 overexpression rescues neuronal differentiation. The dNE cells carrying dox-inducible SOX2 shRNA were transduced with lentivirus expressing GFP or a SOX2-GFP fusion protein (lacking the endogenous SOX2 3′UTR targeted by SOX2-specific shRNA) and cultured under neurogenic conditions in the presence of dox. After 14 days, neuronal differentiation was assayed by Tuj1 immunostaining. Arrows point to TuJ1+ neurons coexpressing exogenous SOX2-GFP. Blue, Hoechst. Area values for a given marker were normalized to total Hoechst area (see Experimental Procedures for details). Scale bars, 100 mm; *p < 0.05, **p < 0.005. Error bars ± SE.
