Table 2.
Indoline-based agonists.
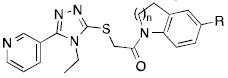
| ||||
|---|---|---|---|---|
| Cmpd | R | n | EC50 (μM)a | % VUAA1 efficacyb |
| 6a (VUAA1) | N/A | 35.1 | 100 % | |
| 7a | H | 1 | - | No agonism |
| 7b | Br | 1 | 21.5 | 82% |
| 7c | F | 1 | - | No agonism |
| 7d | Methyl | 1 | - | No agonism |
| 7e | Ethyl | 1 | 38.6 | 128% |
| 7f | Methoxy | 1 | - | No agonism |
| 7g | N,N-dimethylamino | 1 | - | No agonism |
| 7h | Isopropyl | 1 | - | No agonism |
| 7i | Tertbutyl | 1 | - | No agonism |
| 7j |
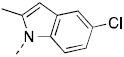 c c
|
47.6 | 71% | |
| 7k | Br | 2 | - | No agonism |
Mean result of 4 experiments.
Maximum agonism of the compound, normalized to the activity of VUAA1.
Entire ring replaced.
