Table 3.
Examination of the pyridyl ring SAR.
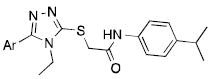
| |||
|---|---|---|---|
| Cmpd | Ar | EC50 (μM)a | % VUAA1 efficacyb |
| 6f | 3-pyridyl | 11.7 | 127 % |
| 8a | H | - | No agonism |
| 8b | Br | - | No agonism |
| 8c | 2-pyridyl | - | No agonism |
| 8d | 4-pyridyl | 6.8 | 150 % |
| 8e | 2-F, 4-Pyridyl | 60.1 | 148 % |
| 8f | 3-pyrrolyl | - | No agonism |
| 8g | 3-furyl | - | No agonism |
| 8h | 3-thiophenyl | - | No agonism |
| 8i | 1-methyl,3-pyrazolyl | - | No agonism |
| 8j | 4-pyrazolyl | - | No agonism |
| 8k | 1-methyl,4-pyrazolyl | 107 | 34 % |
| 8l | 5-thiazolyl | 10.7 | 36 % |
Mean result of 4 experiments.
Maximum agonism of the compound, normalized to the activity of VUAA1.
