Table 4.
Examination of substitution on the triazole nitrogen.
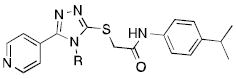
| |||
|---|---|---|---|
| Cmpd | R | EC50 (μM)a | % VUAA1 efficacyb |
| 8d | Ethyl | 6.8 | 150 % |
| 9a | Methyl | - | No agonism |
| 9b | i-Propyl | - | No agonism |
| 9c | t-Butyl | - | No agonism |
| 9d | n-Propyl | 84.0 | 28 % |
| 9e | n-Butyl | - | No agonism |
| 9f | Allyl | 35.9 | 111 % |
| 9g | Cyclopropyl | 3.9 | 162 % |
| 9h | Cyclopentyl | LAc | 13 % |
| 9i | Cyclohexyl | - | No agonism |
| 9j | Phenyl | - | No agonism |
Mean result of 4 experiments.
Maximum agonism of the compound, normalized to the activity of VUAA1.
LA = Low agonism. Compound shows agonism only at the highest concentration tested, but no EC50 could be calculated.
