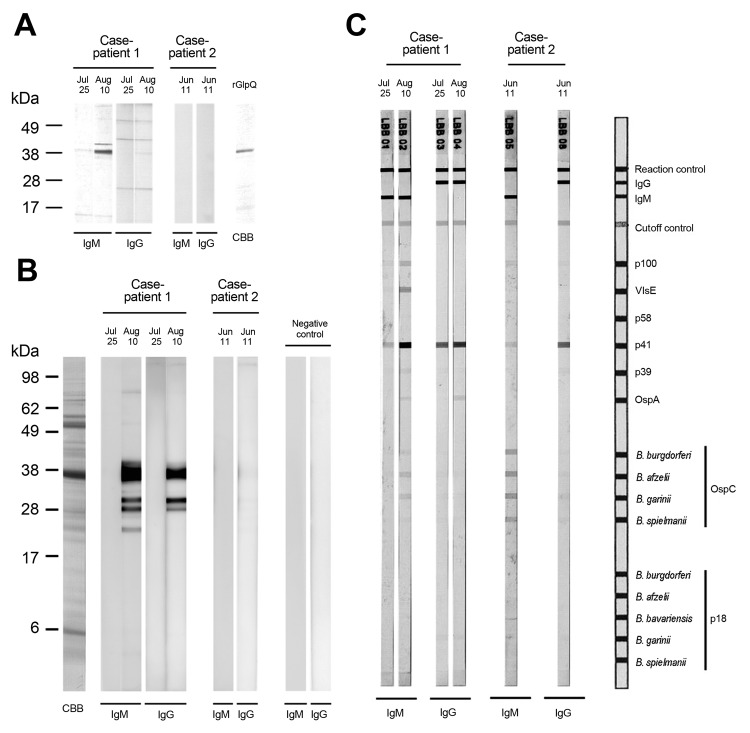
Figure.
Immunoblot analysis of serum reactivity to antigens of Borrelia miyamotoi and Lyme disease borreliae, Japan. Serum samples obtained from 2 patients were examined. For case-patient 1, acute-phase serum obtained on July 25, 2011, and convalescent-phase serum obtained on August 10 were used. For case-patient 2, acute-phase serum obtained on June 11 was used. A) Reactivity to recombinant glycerophosphodiester phosphodiesterase (GlpQ) antigen. Crude rGlpQ were used for immunoblot analysis (10). Recombinant GlpQ was separated by electrophoresis on a 5%–20% polyacrylamide gradient gel (Wako Pure Chemical Industries Inc., Osaka, Japan), and antigen was stained with Coomassie brilliant blue. CBB, protein profile. Molecular mass markers are shown on the left. B) Reactivity of patient serum samples to whole cell lysate of B. miyamotoi antigens. A low-passage strain of B. miyamotoi (strain MYK3) was used for immunoblot analysis (10). A negative control was serum obtained from a healthy human (resident of an area to which Lyme disease was not endemic). Molecular mass markers are shown on the left. C) Serodiagnosis of Lyme disease by immunoblot analysis of serum samples from the 2 patients. OspC, outer surface protein C.