Table 1.
Amination of Benzaldehyde (1a) with Pentafluorophenyl Azide (2a) Catalyzed by [Co(Por)]a
| ||||
|---|---|---|---|---|
| entry | catalyst | temp (°C) | solvent | yield (%)b |
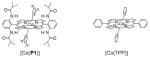
| 1 | [Co(P1)] | 80 | PhH | 56 |
| 2 | [Co(P1)] | 80 | PhCl | 77 |
| 3 | [Co(P1)] | 80 | 4-CF3C6H4Cl | 71 |
| 4 | [Co(P1)] | 80 | PhF | 56 |
| 5 | [Co(P1)] | 80 | hexane | 69 |
| 6 | [Co(P1)] | 80 | ClCH2CH2Cl | 65 |
| 7 | [Co(P1)] | 40 | PhCl | trace |
| 8 | [Co(TPP)] | 80 | PhCl | trace |
| 9 | - | 80 | PhCl | NRc |
| ||||
All reactions were carried out with 1.0 equiv. of aldehyde (0.2 M) and 1.2 equiv. of azide using 3 mol % [Co(Por)] as catalyst.
19F-NMR yields.
NR = no reaction.
