Table 3.
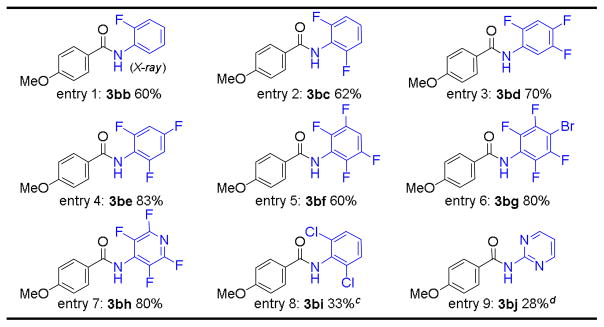
|
All reactions were carried out in PhCl using 3 mol % of [Co(P1)] as catalyst with 1.0 equiv. of aldehyde (0.2 M) and 1.2 equiv. of azide for 24 h at 80 °C.
Isolated yields.
At 100 °C.
The yield was based on 1H NMR.

|
All reactions were carried out in PhCl using 3 mol % of [Co(P1)] as catalyst with 1.0 equiv. of aldehyde (0.2 M) and 1.2 equiv. of azide for 24 h at 80 °C.
Isolated yields.
At 100 °C.
The yield was based on 1H NMR.