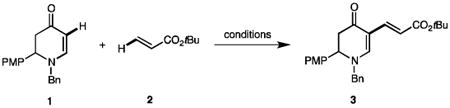
Table 1. Optimization of aerobic dehydrogenative olefination of enaminones.
| |||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Entry[a] | [Pd] (mol %) | [Cu] (mol %) | Atm. | Additive (mol %) | Solvent | Temp (°C) | Yield (%) [b] |
| 1 | Pd(OAc)2 (10) | Cu(OAc)2 (200) | N2 | KTFA (100) | DMF | 80 | 87 (81[c]) |
| 2 | Pd(OAc)2 (10) | – | air | KTFA (100) | DMF | 80 | 27 |
| 3 | Pd(OAc)2 (10) | – | O2 | KTFA (100) | DMF | 80 | 44 |
| 4 | Pd(OAc)2 (10) | Cu(OAc)2 (20) | O2 | KTFA (100) | DMF | 80 | 41 |
| 5 | Pd(OAc)2 (5) | Cu(OAc)2 (5) | O2 | catechol (10) | DMF | 80 | 40 |
| 6 | Pd(OAc)2 (10) | Cu(OAc)2 (10) | O2 | catechol (20) | DMF | rt | 78 |
| 7 | Pd(OAc)2 (10) | Cu(OAc)2 (10) | air | catechol (20) | DMF | rt | 66 |
| 8 | Pd(OAc)2 (10) | Cu(OAc)2 (10) | O2 | catechol (20) | DMSO | rt | 62 |
| 9 | Pd(OAc)2 (10) | Cu(OAc)2 (10) | O2 | catechol (20) | MeCN | rt | 65 |
| 10 | Pd(OAc)2 (10) | Cu(OAc)2 (10) | O2 | catechol (20) | THF | rt | 39 |
| 11 | Pd(TFA)2 (10) | Cu(OAc)2 (10) | O2 | catechol (20) | DMF | rt | 81 |
| 12 | PdCl2 (10) | Cu(OAc)2 (10) | O2 | catechol (20) | DMF | rt | 57 |
| 13 | Pd(TFA)2 (10) | CuCl2 (10) | O2 | catechol (20) | DMF | rt | 27 |
| 14 | Pd(TFA)2 (10) | Cu(OAc)2 (10) | O2 | catechol (20) + 4Å MS | DMF | rt | 91 (89[c]) |
Other conditions: 1 (0.10 mmol), 2 (0.40 mmol), balloon (1 atm), solvent (0.5 mL), 24 h (PMP = para-methoxyphenyl).
1H NMR yields with Ph3SiMe (1.0 equiv) as the internal standard.
Isolated yield. (Detailed optimization is in the Supporting Information.)
