Table 3.
Structure–activity relationships of novel phenytoin derivatives
| Compound name | %BTX | Structure |
|---|---|---|
| Phenytoin (DPH) | 28 | 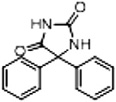 |
| Compound 1 (2a in Supplementary Scheme 1) | 70 | 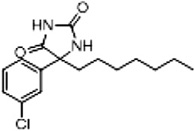 |
| 3a (Supplementary Scheme 1) | 72 | 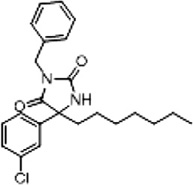 |
| 3d (Supplementary Scheme 1) | 83 | 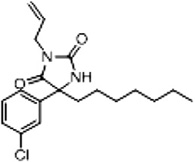 |
| 3c (Supplementary Scheme 1) | 17 | 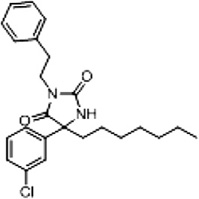 |
| 2b (Supplementary Scheme 1) | 79 | 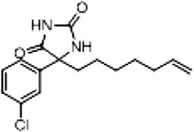 |
| 11a (Supplementary Scheme 2) | 87 | 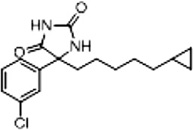 |
| 11b (Supplementary Scheme 2) | 28 | 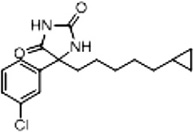 |
| Compound 2 (Scheme 2 and Supplementary Scheme 5) | 86 | 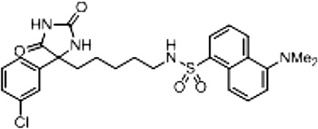 |
%BTX is the percent displacement of [3H] batrachotoxin-20-α-benzoate (BTX) from the saturated site 2 of VGSCs in rat brain synaptoneurosomes at 40 µM of test compound. Compounds that displaced greater than 50% of the [H] BTX-B were considered active analogs for further study.
