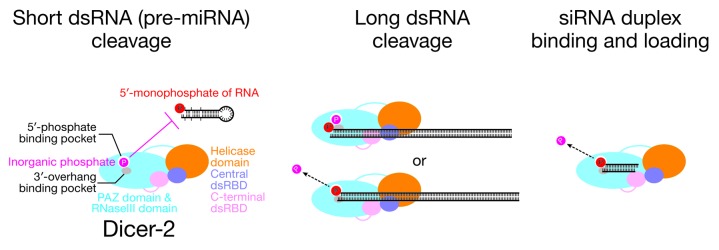
Figure 6. Models for the inhibition by inorganic phosphate of fly Dicer-2 processing of short, but not long, dsRNA and the role of the phosphate-binding pocket in siRNA loading. The PAZ domain of fly Dicer-2 has binding pockets for the 5′-monophosphate and the 2-nucleotide 3′-overhang of a pre-miRNA substrate. An inorganic phosphate molecule occupies the binding pocket in place of the 5′-monophosphate of the pre-miRNA, blocking its binding. Long dsRNA is recognized mainly by the helicase domain and/or the dsRBD domains and does not need a specific terminal structure to be efficiently cleaved. Long dsRNA is not blocked by the bound inorganic phosphate, since the strong interaction with the helicase domain or the dsRBD domains allows it either to bind tightly enough to Dicer-2 without additional interactions with the PAZ domain or to displace the inorganic phosphate from the binding pocket. The phosphate-binding pocket binds 5′-monophosphate of siRNA duplex in the loading step. The bound inorganic phosphate is displaced by the siRNA.
