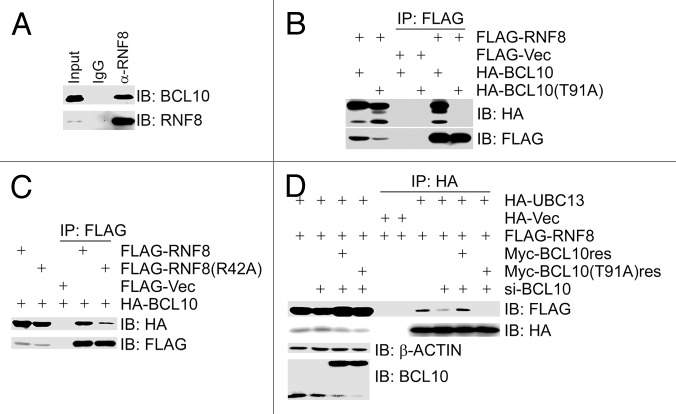
Figure 3. BCL10 functions as an assembly factor for the RNF8–Ubc13 complex in a phosphorylation-dependent manner. (A) BCL10 interacted with RNF8. Total cell lysates were extracted from 293T cells, immunoprecipitated and immunoblotted with antibodies as indicated. (B) ATM-mediated phosphorylation of BCL10 on T91 was required for its binding to RNF8. 293T cells were co-transfected with FLAG-RNF8 and HA-BCL10 or HA-BCL10(T91A). Total cell lysates were extracted 48 h later, immunoprecipitated, and immunoblotted with antibodies as indicated. (C) The FHA domain of RNF8 was necessary for its binding to BCL10. 293T cells were co-transfected with HA-BCL10 and FLAG-RNF8 or the FHA domain mutant FALG-RNF8(R42A). Total cell lysates were extracted 48 h later, immunoprecipitated, and immunoblotted with antibodies as indicated. (D) Depletion of BCL10 by siRNA reduced the interaction between RNF8 and UBC13. BCL10-depleted 293T cells were co-transfected with HA-UBC13, FLAG-RNF8, and siRNA-resistant MYC-BCL10res or MYC-BCL10(T91A)res. Total cell lysates were extracted 48 h after co-transfection, immunoprecipitated, and immunoblotted with antibodies as indicated.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.