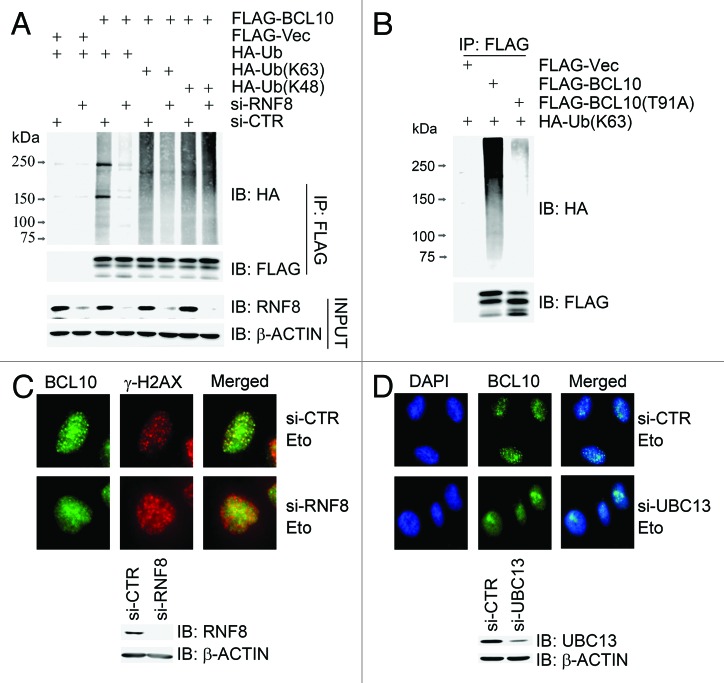
Figure 4. K63-linked ubiquitination of BCL10 is mediated by RNF8–UBC13 complex. (A) BCL10 is modified with K63-linked ubiquitins. RNF8-depleted or mock-depleted 293T cells were co-transfected with FLAG-Vec or FLAG-BCL10 along with HA-ubiquitin, HA-ubiquitin K48 only (HA-Ub[K48[), or HA-ubiquitin K63 only (HA-Ub[K63]). Total cell lysates extracted 48 h after transfection were subjected to immunoprecipitation with an anti-FLAG antibody and immunoblotting with antibodies as indicated. (B) BCL10(T91A) was not ubiquitinated in vivo. 293T cells were transiently co-transfected with HA-ubiquitin and FLAG-Vec, FLAG-BCL10, or FLAG-BCL10(T91A). Immunoprecipitates with an anti-FLAG antibody were probed with antibodies as indicated. (C) Depletion of RNF8 reduced etoposide-induced focus formation of BCL10. HeLa cells were transfected twice with either RNF8 siRNAs or a non-targeting control siRNA (si-CTR). Forty-eight hours after the second transfection, cells were treated with etoposide for 1 h before they were fixed with 4% paraformaldehyde. Immunofluorescence staining with anti-BCL10 and anti-γ-H2AX was performed as described in the Methods. The efficiency of RNF8 knockdown was determined by immunoblotting with antibodies as indicated. Scale bars: 20 μM. (D) Depletion of UBC13 reduced etoposide-induced focus formation of BCL10. Experiments were performed as described in (C) except that UBC13 was depleted in HeLa cells.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.