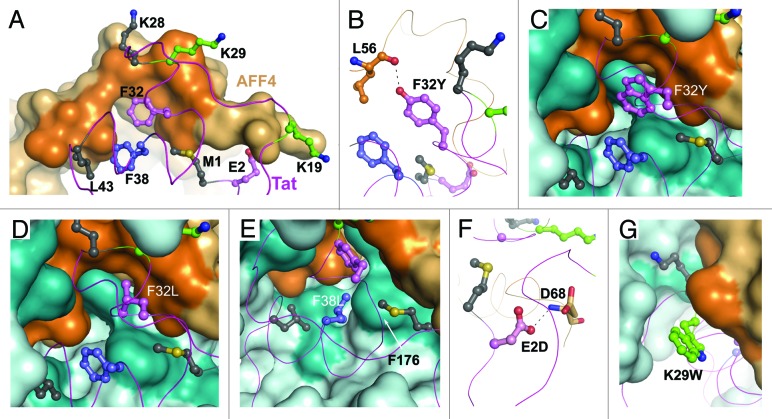
Figure 4. Structural constraints for Tat mutation imposed by AFF4. (A) Color-coded AFF4-interacting residues of Tat: invariant residues are in deep gray, residue with a randomly occurring polymorph is in slate, residues with predominantly functionally equivalent substitutions are in pink, and residues with a variety of substitutions are in green. AFF4 surface is shown in light orange. Darker orange surface area corresponds to Tat-interacting hydrophobic residues of AFF4. (B) Tat Phe32Tyr substitution results in a H-bond to main-chain oxygen of AFF4 Leu56. (C) Tat Phe32Trp substitution inserts tryptophan side chain into the hydrophobic cavity surrounded by Tat, Cyclin T1 and AFF4 residues. (D) Tat Phe32Leu substitution shows an empty space on the left of Leu32 side chain. (E) Tat Phe38Leu substitution places Leu38 side chain close to the side chain of Cyclin T1 Phe176. (F) Tat Glu2Asp substitution results in a H-bond formation between Asp2 and Asp 68 of AFF4. (G) Lys29 of Tat tolerates a variety of substitutions. This panel displays an example of Lys29 substitution to a bulky tryptophan side chain.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.