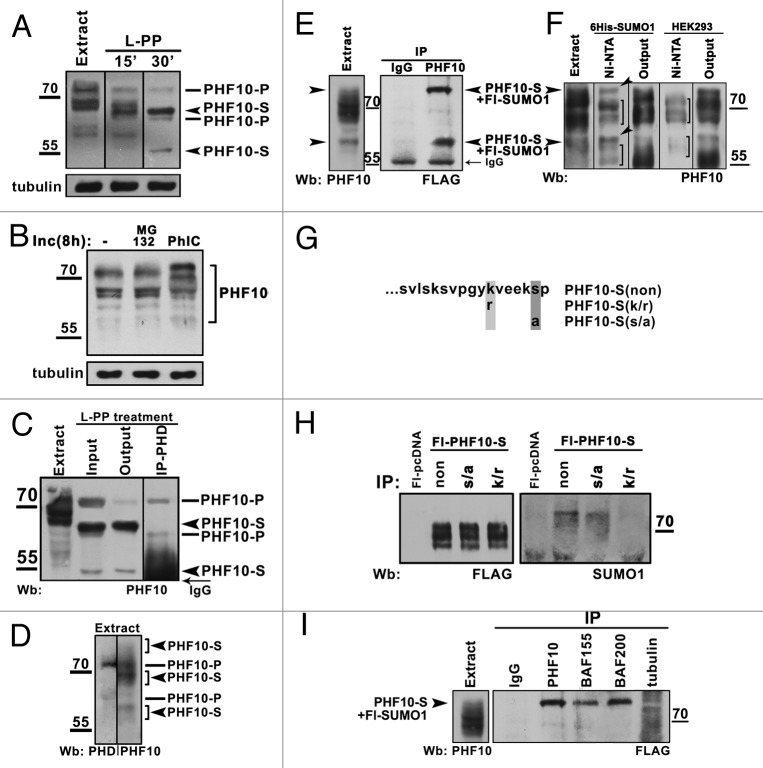
Figure 2. Post-translational modifications of PHF10 isoforms. (A) Treatment of HEK293 cell extract with lambda protein phosphatase (L-PP) for 15 or 30 min leads to reduction of numerous PHF10 isoforms to 4 stable protein bands (arrowheads). (B) Inhibition of phosphatases stabilizes high-molecular-weight PHF10 bands. Western blotting of PHF10 in protein extracts from wild-type HEK293 cells and from HEK293 cells grown for 8 h in the presence of MG132 proteasome inhibitor or phosphatase inhibitor cocktail (PhIC). Accumulation of high-molecular-weight PHF10 bands (arrowheads) can be seen in cells grown in the presence of PhIC. (C) Immunoprecipitation of PHD-containing PHF10 forms (PHF10-P) performed with anti-PHD antibodies from the HEK293 extract treated with L-PP. The PHF10-S isoforms remain in the output material. (D) Western blotting of HEK293 extract with anti-PHD or anti-PHF10 (ab1) antibodies. The PHF10-P and PHF10-S isoforms are indicated. (E) Endogenous PHF10-S isoforms modified by SUMO 1 conjugation. FLAG-tagged SUMO 1 was transiently expressed in HEK293 cells, and the cell extract was immunoprecipitated by anti-PHF10 antibodies. Western blotting of the precipitate with anti-FLAG antibodies revealed 2 sumoylated PHF10 forms. Their electrophoretic mobility coincided with that of modified PHF-S isoforms in the HEK293 extract (left panel, indicated by arrowheads). (F) Nuclear extract from HEK293 cells transiently transfected with 10 His- SUMO 1 or from control (nontransfected) HEK293 cells was incubated with Ni-NTA, and precipitated proteins were resolved by western blotting with antibodies against PHF10. Sumoylated PHF10 bound by Ni-NTA resin (arrows) was depleted from the pool of PHF10 isoforms (see “Output”). The PHF10 forms indicated with brackets are those nonspecifically bound by Ni-NTA. (G) The amino acid sequence of wild-type and mutated PDSM. In mutants, either serine was replaced by alanine or lysine was replaced by arginine to prevent phosphorylation or sumoylation, respectively. (H) The PDSM motif of PHF10-S conjugates SUMO 1 in a phosphorylation-dependent manner. FLAG-tagged wild-type and mutated forms of PHF10-S were transiently expressed HEK293 cells and purified on anti-FLAG agarose. The recombinant PHF10 was precipitated with anti-FLAG antibodies from the cell extract, and equal amounts of precipitate were loaded onto a gel (FLAG). Staining with anti-SUMO 1 antibody revealed sumoylated recombinant PHF10 (SUMO 1). (I) The sumoylated PHF10-S is associated with PBAF. Immunoprecipitation with antibodies against BAF200 or BAF155 was performed from the extract of HEK293 cells stably transfected with FLAG-tagged SUMO 1. The western blot was developed with antibodies against FLAG to detect sumoylated PHF10 (arrowhead). Immunoprecipitation with antibodies against tubulin was used as a negative control.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.