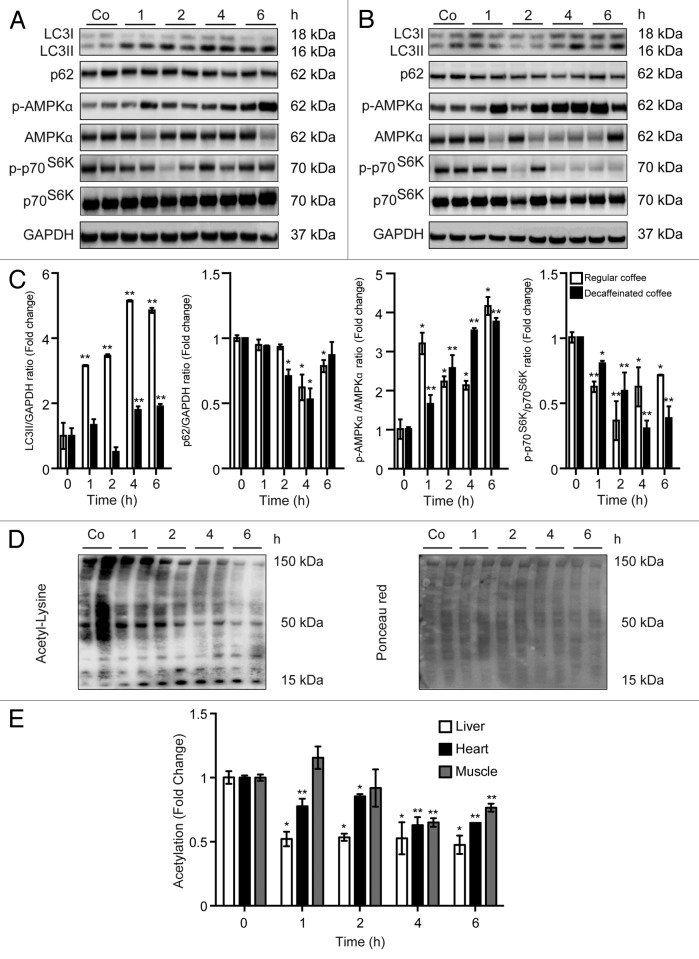
Figure 2. Short-term administration of both regular coffee and decaffeinated coffee induces autophagy accompanied by a reduction in global acetylation levels of proteins in the liver. (A and B). Immunoblotting analysis of short-term coffee administration on autophagy regulation in liver. Gavage of both regular coffee (A) and decaffeinated coffee (B) resulted in an activation of autophagy, although at different extent and timing, as measured by LC3 lipidation and p62 degradation (quantified in C). In both cases, autophagy induction was accompanied by an activation of AMPK and by a reduction in the activity of mTORC1, as measured by the phosphorylation of its substrate p70s6k (quantified in C). Representative images are depicted in (AandB). Results from n = 3 independent experiments are presented as fold change ± SEM *P < 0.05; **P < 0.01; (unpaired, 2-tailed Student t test), compared with untreated mice. (D and E) Immunoblot detection of protein acetylation in mice administered with regular coffee. Coffee administration (by gavage) resulted in a significant drop in the overall acetylation levels of proteins in liver, heart, and muscle (quantified in E) in a range of time of 1 to 6 h depending on the tissue. Panels in (D) refer to liver. Ponceau red staining was used to monitor equal loading of the lanes. Results from n = 3 independent experiments are presented as fold change ± SEM *P < 0.05; **P < 0.01 (unpaired, 2-tailed Student t test), compared with untreated mice.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.