Abstract
Background
The choice of the optimal benzodiazepine to treat prehospital status epilepticus is unclear. Lorazepam is preferred in the emergency department, but concerns about nonrefrigerated storage limits emergency medical services (EMS) use. Midazolam is increasingly popular, but its heat stability is undocumented.
Objective
This study evaluated temperature-dependent degradation of lorazepam and midazolam after 60 days in the EMS environment.
Methods
Lorazepam or midazolam samples were collected prior to (n = 139) or after (n = 229) 60 days of EMS deployment during spring–summer months in 14 metropolitan areas across the United States. Medications were stored in study boxes that logged temperature every minute and were stored in EMS units per local agency policy. Mean kinetic temperature (MKT) exposure was derived for each sample. Drug concentrations were determined in a central laboratory by high-performance liquid chromatography. Concentration as a function of MKT was analyzed by linear regression.
Results
Prior to deployment, measured concentrations of both benzodiazepines were 1.0 relative to labeled concentration. After 60 days, midazolam showed no degradation (mean relative concentration 1.00, 95% confidence interval [CI] 1.00–1.00) and was stable across temperature exposures (adjusted R2 –0.008). Lorazepam experienced little degradation (mean relative concentration 0.99, 95% CI 0.98–0.99), but degradation was correlated to increasing MKT (adjusted R2 0.278). The difference between the temperature dependence of degradation of midazolam and lorazepam was statistically significant (T = −5.172, p < 0.001).
Conclusions
Lorazepam experiences small but statistically significant temperature-dependent degradation after 60 days in the EMS environment. Additional study is needed to evaluate whether clinically significant deterioration occurs after 60 days. Midazolam shows no degradation over this duration, even in high-heat conditions.
Keywords: midazolam, lorazepam, temperature, emergency medical services
Introduction
Prolonged seizures frequently require treatment by emergency medical services (EMS) prior to hospital arrival, and benzodiazepines are the mainstay of initial therapy.1,2 However, the optimal agent for use by paramedics in the prehospital setting is unclear. Lorazepam, considered the benzodiazepine of choice for seizure cessation in the hospital environment,3 is generally thought to require replacement after 60 days unless it is refrigerated.1 This need for refrigerated storage has prevented lorazepam's widespread adoption for prehospital use, despite demonstrated effectiveness in this setting.1 Diazepam experiences degradation similar to lorazepam and is no better than midazolam in control of status epilepticus.4,5 As such, midazolam is becoming more widely used by EMS because it is thought to be heat-stable and can be readily given intramuscularly or intranasally if needed. However, its use for seizures is an off-label indication that has not been approved by the Food and Drug Administration,6 and its temperature stability has not yet been established.
Although product labeling for lorazepam calls for refrigerated storage, the rate of medication degradation without refrigerated storage, and the extent to which this degradation is temperature-dependent, is poorly documented. In a single-site EMS study with mild environmental temperatures and nonrefrigerated storage of the drug, lorazepam concentration did not substantially degrade over a 60-day period; lorazepam stored in an oven kept at 37°C experienced significant degradation, suggesting that lorazepam's stability is heat-sensitive.4 Midazolam is thought to be stable at room temperature, but the heat stability and degradation at varying storage temperatures in the prehospital setting are poorly documented.
Storage conditions for EMS drugs are known to vary from the United States Pharmacopeia (USP) standard for storage at “controlled room temperature.”7,8 However, the effect of typical EMS storage temperatures on drug degradation is poorly understood.8 Determining the heat stability of benzodiazepines is an important step in ensuring clinical effectiveness and patient safety and will be helpful to EMS planners when choosing which medications to stock, rotate, retire, and replace.
The objective of this investigation was to provide empirical data on the magnitude and temperature dependency of degradation in concentrations of lorazepam and midazolam over 60 days of EMS storage at sites with large variations in ambient temperatures.
Methods
Study Design and Setting
This was an experimental in-field pharmacostability study conducted during a multicenter, randomized, controlled trial of paramedic treatment of status epilepticus with lorazepam or midazolam.2
As part of the multicenter trial, midazolam and lorazepam were distributed to EMS agencies in 14 cities across the United States. Midazolam (5 mg/mL) was packaged in a glass cartridge within an autoinjector (Meridian Medical Technologies, Columbia, MD) and lorazepam (2 mg/mL) was packaged in a prefilled disposable single-use glass syringe (Carpuject, Hospira, Lake Forest, IL). Study medications were stored in instrumented boxes that recorded temperature every minute for 60 days or until the medication was used, whichever came first. Unused study drugs were retired at 60 days.
For quality assurance and research purposes, two subsets of sample drug kits were randomly selected by a computerized drug-tracking system for analysis. A baseline subset was drawn from refrigerated new kits that were never placed in the field. The other was a subset of retired, unused study drugs collected after 60 spring or summer days in the field between April and August 2010. For the purpose of this investigation, the two participating study sites that are known to have the highest ambient temperatures were deliberately oversampled by a ratio of 3:1 to obtain a larger number of samples expected to experience high-heat conditions.
Generally, EMS agencies stored the medications in the study boxes alongside other routine medications and per individual agency policies. Use of temperature-control systems beyond normal vehicle air conditioning was not specified in the study protocol. The drugs were stored inside the cabs or external compartments of fire apparatus, ambulances, and other vehicles used for emergency medical response. Some vehicles were kept in environmentally controlled stations unless responding to an emergency call, while others were in constant exposure to ambient temperatures during work shifts.
Sample size was estimated to provide significance of 0.05 and power 0.8, assuming a mean difference of 2.5% for the aggregate comparison of midazolam and lorazepam groups and a within-group sample variability (standard deviation) of 5%.
Methods of Measurement
Temperature was measured every minute for 60 days by a microprocessor-controlled thermistor in each study box and recorded to a nonvolatile memory card (MicroSD, SanDisk, Milpitas, CA). This time-varying signal was analyzed and summarized by determination of mean kinetic temperature (MKT). MKT is a metric used to describe the overall effect of temperature fluctuations on heat-sensitive materials and is used widely in the pharmaceutical industry.9 This measure is not simply a weighted average of temperature, but serves as a way to express the cumulative heat stress to which a product has been exposed over time. The MKT was calculated for each sample across 60 days of temperature data (Stability System II software, Scien Tek Software, Tustin, CA). Daily ambient temperature data were gathered from online resources for each city.10
Samples were analyzed in a commercial laboratory (DynaLabs, St. Louis, MO) by high-performance liquid chromatography (HPLC) to determine concentration of the active drug. Samples were refrigerated (including shipping) before deployment in the field and after retirement prior to analysis to prevent further degradation.
Data Collection and Processing
Memory cards from the selected study boxes were sent to the study's clinical coordinating center (CCC), and drug samples were sent directly to the processing center for analysis. Results of the HPLC were returned to the CCC to be paired with temperature data. Data were managed using Microsoft Excel (Microsoft Corp., Redmond, WA) and analyzed via SPSS version 19 (International Business Machines, Armonk, NY).
Outcome Measures
The primary outcome was relative reduction in medication concentration from the labeled concentration.
Primary Data Analysis
Linear regression determined the dependence of each medication's degradation as a factor of MKT. The beta coefficient was calculated to compare the regression slopes, as a means to compare the differences in the relationship between MKT and degradation for midazolam and lorazepam.11
Results
Baseline
Prior to study drug deployment, 139 randomly selected samples were sent for baseline concentration determination by HPLC as part of the larger trial's quality assurance process. These samples were kept refrigerated, not exposed to prolonged periods of ambient temperature prior to testing, and confirmed to have baseline concentrations consistent with manufacturer labeling. The mean relative concentration (actual versus labeled) at baseline was 1.00 (95% confidence interval [CI] 0.99–1.00) for midazolam (n = 74) and 1.01 (95% CI 1.01–1.01) for lorazepam (n = 65).
Between April and August 2010, 122 midazolam and 107 lorazepam samples were randomly selected from unused study drugs of 14 cities and sent for HPLC analysis after 60 days of environmental exposure in EMS vehicles. The mean monthly ambient temperatures for the 14 cities ranged from 9.2°C to 35.9°C (Table 1).
Table 1. Mean Monthly and Aggregate Ambient Temperatures (°C) and Mean Kinetic Temperatures (°C) of Medication Samples for the 14 Cities During the Study Period.
| City | April | May | June | July | August | Aggregate | MKT | Samples |
|---|---|---|---|---|---|---|---|---|
| 1 | 28.1 | 25.7 | 32.3 | 35.9 | 34.4 | 30.9 | 21.7 | 19 |
| 2 | 14.8 | 18.9 | 23.9 | 25.5 | 25.6 | 21.5 | 21.5 | 6 |
| 3 | 18.4 | 23 | 27.4 | 27.9 | 28.2 | 24.7 | 22.3 | 6 |
| 4 | 12.3 | 17.1 | 21.9 | 24.8 | 24 | 19.8 | 18.2 | 12 |
| 5 | 15.2 | 19.3 | 24.8 | 25.6 | 25.6 | 21.8 | 22.7 | 6 |
| 6 | 12.7 | 15.9 | 20.7 | 24.9 | 25 | 19.6 | 25.0 | 5 |
| 7 | 12.1 | 19.6 | 25.7 | 27.6 | 26.1 | 22.0 | 20.2 | 5 |
| 8 | 9.2 | 10.6 | 13.5 | 15.3 | 15.7 | 12.7 | 22.4 | 6 |
| 9 | 11.2 | 13.7 | 19.3 | 18.6 | 18.8 | 16.1 | 21.0 | 6 |
| 10 | 12.9 | 17.6 | 21.6 | 24.2 | 23.6 | 19.8 | 25.3 | 6 |
| 11 | 20.8 | 26.3 | 29.3 | 28.9 | 30.7 | 26.9 | 23.6 | 22 |
| 12 | 11.2 | 13.7 | 19.3 | 18.6 | 18.8 | 16.1 | 17.9 | 6 |
| 13 | 16.8 | 21.2 | 27.3 | 28.2 | 26.6 | 23.7 | 24.8 | 6 |
| 14 | 13.8 | 18.5 | 24.6 | 26.7 | 24.9 | 21.5 | 22.3 | 6 |
MKT = mean kinetic temperature.
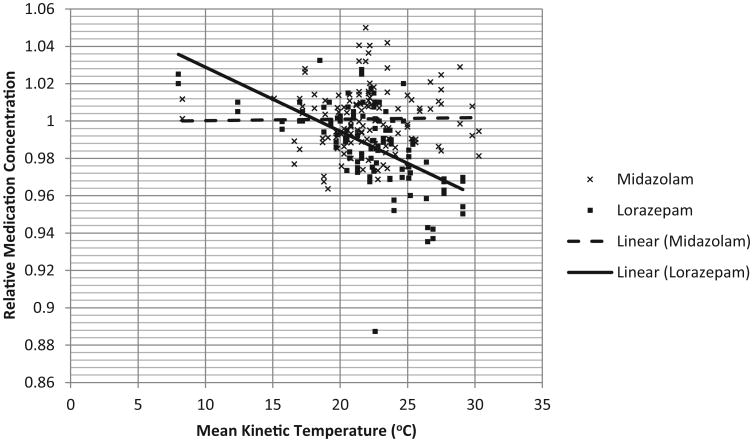
The mean MKT was 22.1°C (95% CI 21.6°C–22.5°C) for all samples. There was no difference in the overall MKT for midazolam or lorazepam samples. Figure 1 shows relative drug concentrations over the range of individual MKT exposures. Midazolam showed no significant degradation over time (mean relative concentration 1.00, 95% CI 1.00–1.00); degradation over time was not correlated with temperature (adjusted R2 –0.008), demonstrating stability over a broad range of temperatures. Lorazepam experienced some degradation (mean relative concentration 0.99, 95% CI 0.98–0.99), and greater degradation was correlated with increasing MKT (adjusted R2 0.278), suggesting that the degree of degradation is affected by the degree of exposure to higher temperatures.
Figure 1.
Relative medication concentration versus mean kinetic temperature for midazolam and lorazepam after 60 days of heat exposure in active emergency medical services vehicles. Individual data points and the linear trend lines are displayed.
The absolute difference in mean concentration of midazolam and lorazepam was small but statistically significant (1.4%, p < 0.001). However, increasing MKT had a much greater effect on lorazepam than midazolam (T = −5.172, p < 0.001), providing evidence that lorazepam is more heat-sensitive than midazolam.
Discussion
Both lorazepam and midazolam maintained clinically acceptable concentrations of active drug after 60 days of EMS deployment. Lorazepam demonstrated a small amount of temperature-dependent degradation over this interval, whereas midazolam did not.
There is evidence that not all medications used by EMS are affected by storage in uncontrolled environments.12 However, understanding which drugs are affected, and to what degree, is essential to ensure that patients receive effective treatment. Prior to this study, the heat stability of midazolam in the EMS environment had not been reported. We demonstrated that after 60 days of environmental exposure, midazolam shows little degradation over a broad range of temperature exposures. Lorazepam, on the other hand, shows some temperature-dependent degradation. The stability of both medications over a longer period of exposure deserves investigation. The ambient temperatures encountered in this study were in the cool (8°C–15°C), room-temperature (15°C–30°C), and warm (30°C–40 °C) ranges as defined by the USP.9 The effects of excessive heat (>40°C) require further study.
Our results build upon those previously reported,4 since this investigation occurred in more diverse environments, thereby allowing further evaluation of the degree to which observed drug degradation was affected by MKT exposure. The median lorazepam degradation we observed was similar to that observed by Gottwald et al.,4 and the amount of degradation is indeed tied to increasing MKT. The clinical implications of lorazepam's degradation are uncertain. However, it is notable that the concentrations in some lorazepam samples (5/106, 4.7%) were reduced by more than 5% when they came from high-heat environments. Thus, the deterioration of lorazepam may be rapid and considerable in areas that routinely experience extreme temperatures, such as the deep southern and southwestern regions of the United States.
The two cities with the highest ambient temperatures were purposely oversampled; however, ambient temperatures and sample MKT did not directly correlate (Table 1). This unexpected observation may be related to EMS system operational aspects, whereby medications kept in garaged fire department bays likely have better temperature controls than dynamically deployed third-service EMS agencies. When considering the potential impact of heat exposure on medications, EMS agencies should sample the temperatures where drugs are stored (i.e., ambulance patient compartment) and not rely solely on ambient temperature.
Limitations and Future Research
Baseline and postexposure measurements were not taken from the same samples. Thus, it is possible that packaged concentrations were different and resulted in systemic bias. However, this is unlikely since deployed medications were packaged 1) by a commercial vendor with extensive product quality control, 2) according to USP recommendations, and 3) in the same manner as the samples used to determine baseline concentrations (which all had very small variability).
The use of advanced temperature-control systems by participating agencies was not captured as part of data collection; however, the temperatures recorded for samples do not suggest the use of refrigerators. Further investigation of drug-storage strategies used by agencies where the observed MKT is different from observed ambient temperatures could provide evidence in support of best practices.
Conclusion
These data confirm that lorazepam experiences small but statistically significant temperature-dependent degradation after exposure to 60 days in the EMS environment. Additional study is needed to evaluate whether clinically significant deterioration occurs after 60 days. Midazolam does not show any sign of degradation over this duration, even in high-heat conditions.
Acknowledgments
This work was conducted as part of the RAMPART trial, which was supported by awards from the National Institute of Neurological Disorders and Stroke (NINDS) (U01NS056975 and U01NS059041); the National Institutes of Health Office of the Director CounterACT Program; and the Biomedical Advanced Research and Development Authority of the Assistant Secretary for Preparedness and Response.
Appendix
The Neurological Emergencies Treatment Trials investigators are: Clinical Coordinating Center
Robert Silbergleit, MD, Daniel Lowenstein, MD, William Barsan, MD, Arthur Pancioli, MD, Valerie Stevenson, BAS, RRT, CCRP, Erin Zaleski, MA, Deneil Harney, MPH, MSW, Donna Harsh, MS, Joy Pinkerton, BSN, RN, MS, Allison Kade, BA, Nicholas Siewert, BA, Ashley Pinawin, BS, Catherin Ring, Phebe Brenne
National EMS Coordinator
Kay Vonderschmidt, MPA, MS-EM, NREMT-P
Statistical Data Management Center
Valerie Durkalski, PhD, Yuko Palesch, PhD, Catherine Dillon, Keith Pauls, Qi Wu, Wenle Zhao, PhD
National Institutes of Health
Robin Conwit, MD, Scott Janis, PhD, David Jett, PhD, Brandy Fureman, PhD
Hubs
Wayne State University
Hub Principal Investigator: Robert D. Welch, MD, MS
Primary Study Coordinators: Lynnmarie Mango, MPH, Valerie H. Mika, MS
EMS Director(s)/Coordinator: Jenny Atas, MD
Other Site Investigators: Robert Dunne, MD, Douglas Wheaton, MD, Phillip Levy, MD, MPH, Marc-Anthony Velilla, MD, Robert Sherwin, MD, Brian O'Neil, MD, Angela Groves, MD, Marc Rosenthal, DO, PhD
Participating EMS Service: Detroit EMS
University of Cincinnati
Hub Principal Investigator: Arthur Pancioli, MD
Primary Study Coordinators: Irene Ewing, RN, BSN, Peggy Waymeyer, RN
EMS Director(s)/Coordinator: M. Kay Vonderschmidt, MPA, MS-EM, NREMT-P, Jason McMullan, MD
Other Site Investigators: Hamilton Schwartz, MD, Brian Stettler, MD, William Knight, MD, Opeolu Adeoye, MD, Rhonda Cadena, MD, Jordan Bonomo, MD, Erin Grise, MD, Laura Heitsch, MD, George Shaw, MD, Nick Gagai, CCRP, Pamela Schmit, RN BSN, Sara Stark, Med, Traci Doellman, RN
Participating EMS Services: Cincinnati Fire Department, BlueAsh Fire Department, Forest Park Fire Department, Green Township Fire Department, Florence Fire Department, Independence Fire Department
University of California San Francisco
Hub Principal Investigator: J. Claude Hemphill, III, MD, MAS
Primary Study Coordinators: Michele Meeker, RN, BSN, Kelley Rosborough, BA
EMS Director(s)/Coordinator: Jeany Duncan EMT-P
Other Site Investigators: Karl Sporer, MD, FACEP, FACP, Alan Gelb, MD; Wade Smith, MD, PhD, Prasanthi Ramanujam, MD, Kazuma Nakagawa, MD, Asma Moheet, MD, Hooman Kamel, MD, Bharath Naravetla, MD, Mary Mercer, MD, Christine Wong, MD
Participating EMS Services: San Francisco Fire Department, EMS Division
University of Texas -Houston
Hub Principal Investigator: Elizabeth Jones, MD
Trial Principal Investigator: Truman J. Milling, MD
Primary Study Coordinators: Misty Ottman, RN, BSN, Ben King, Laura LaChance
EMS Directors/Coordinators: Jeff Brockman, RN, Pete Didonato, EMT-P
Other Site Investigator: Paul Hinchey, MD
Participating EMS Service: Austin-Travis County EMS
Emory University
Hub Principal Investigator: David W. Wright, MD
Trial Principal Investigators: Matthew D. Bitner, MD, Gerald W. Beltran, DO
Primary Study Coordinator: Harriet Nevarez, RN, CCRC
EMS Director/Coordinator: Rachel Barnhard, Andrea G. McDougal
Other Site Investigators: Jeffrey F. Linzer Sr, MD, Lisa H. Merck, MD MPH, Tamara Espinoza, MD
Participating EMS Service: Grady EMS
Henry Ford Health System
Hub Principal Investigator: Christopher A. Lewandowski, MD
Trial Principal Investigator: Taher T. Vohra, MD
Primary Study Coordinators: Paula L. Crouse, RN, BSN., MA., Anna E. Baker, RN, BSN
EMS Director/Coordinator: Dean R. Creech EMT-P, I/C
Other Site Investigator: Andrew N. Russman, DO, Joseph B. Miller, MD, Jumana Nagarwala, MD, Daniel J. Miller, MD, Raymond Fowkes, MD, Anne Marie Lundell, RN, BSN
Participating EMS Services: Detroit EMS, West Bloom-field Fire and EMS Services
Stanford University
Hub Principal Investigator: James V. Quinn, MD, MS
Primary Study Coordinators: Stephanie Casal. RN, CNS, Anke Hebig, Mark Liao
EMS Director/Coordinator: Peter D'Souza, MD
Participating EMS Services: Palo Alto Fire Department, San Jose Fire Department, Redwood City Fire Department, San Mateo Fire Department
University of Arizona
Hub Principal Investigator: Kurt R. Denninghoff, MD
Trial Principal Investigator: Daniel W. Spaite, MD
Primary Study Coordinator: Bruce Barnhart, RN, CEP
EMS Director(s)/Coordinator: Willie Haro, CEP
Other Site Investigator: Bentley J. Bobrow, MD
Participating EMS Service: Glendale Fire Department
Virginia Commonwealth University
Hub Principal Investigator: Joseph P. Ornato, MD
Primary Study Coordinator: Sallie L. Noe, RN
EMS Director/Coordinator: Alan D. Payne, CCEMTP
Other Site Investigators: Alan R. Towne, MD, Michael C. Kurz, MD, John T. Carmack, MD
Participating EMS Service: Richmond Ambulance Authority
University of Minnesota
Hub Principal Investigator: Michelle Biros, MD
Trial Principal Investigator: Brian Mahoney, MD
Primary Study Coordinators: Corey Sargent, Kathleen Miller, BSN, CCRC
Other Site Investigators: David Hildebrandt, Chris Kummer, Doug Gesme
Participating EMS Services: Hennepin County EMS
Medical College of Wisconsin
Hub Principal Investigator: Tom P. Aufderheide, MD
Primary Study Coordinator: Joseph T. Brandt Jr., BS, EMT-P
EMS Director/Coordinator: M. Riccardo Colella, DO
Other Site Investigators: Ron Pirrallo, MD, MHSA, Walter Bialkowski, MS, Benjamin Hermanson, BS, Christopher Sandoval, BS, EMT-P, Kevin Morrow, MFA, Kelly McCormick, BS, MBA, Katherine Burpee, BA, Geri Price, BS, Dawn Kawa, BA
Participating EMS Services: Milwaukee County EMS, Milwaukee Fire Department, Franklin Fire Department, Greenfield Fire Department, North Shore Fire Department, Oak Creek Fire Department, South Milwaukee Fire Department, Wauwatosa Fire Department, West Allis Fire Department
University of Kentucky
Hub Principal Investigator: Roger L. Humphries, MD
Primary Study Coordinator: Linda Dechtenberg, RN, BSN, CCRC
EMS Director/Coordinator: Christofer Sweat
Other Site Investigator: L.Creed Pettigrew, MD, MPH
Participating EMS Service: Lexington-Fayette Urban County Government Division of Fire & Emergency Services
University of Pennsylvania
Hub Principal Investigator: Jill M. Baren, MD, MBE Trial Principal Investigator: R. Daniel Bledsoe, MD
Primary Study Coordinator: Barbie Stahlman, MS, Katherine Lamond, BA, Pamela G. Nathanson, MBE
Other Site Investigator: Scott E. Kasner, MD, MSCE, Peter D. Le Roux, MD
Participating EMS Services: York Hospital Medic 97, White Rose Ambulance, Grantley Fire Company, Jacobus Lions Ambulance Club, West York Ambulance
Oregon Health & Science University
Hub Principal Investigators: Craig R. Warden, MD, MPH, Robert A. Lowe, MD, MPH
Primary Study Coordinator: Rachel N. Stone, CCRP
Participating EMS Service: Clackamas Fire District #1
New York Presbyterian Hospital
Hub Principal Investigator: Stephan Mayer, MD, FCCM
Trial Principal Investigator: Neal Flomenbaum, MD
Primary Study Coordinators: M. Cristina Falo, PhD, Lisa-Vanessa Magitbay, RN, Chirag Surti
EMS Directors/Coordinators: Heidi Cordi, MD, Daniel Ribaudo
Other Site Investigators: Axel Rosengart, MD, PhD, Matthew Vibbert, MD, Santiago Ortega-Gutierrez, MD, H. Alex Choi, MD, Emily Gilmore, MD, Rishi Malhotra, MD, Lawrence Berger
Participating EMS Services: New York Presbyterian
Temple University
Hub Principal Investigator: Nina T. Gentile, MD Trial
Principal Investigators: Alvin Wang, DO, Christopher Vates, MD, Ben Usatch, MD
Primary Study Coordinators: Brent B. Freeman, Stacey L. Cleary
Participating EMS Services: Volunteer Medical Services Corps of Lower Merion and Narberth (Narberth Ambulance), Life Lion EMS
University of Maryland
Hub Principal Investigator: Barney Stern, MD
Trial Principal Investigators: Tricia Ting, MD, Gregory Krauss, MD
Primary Study Coordinators: Virginia Ganley, RN, Susan Rice, RN, Jennifer Ronald
EMS Director/Coordinator: Michelle Stevens, RN
Other Site Investigators: Brian Browne, MD, Robert Rosenthal, MD, Peter Hill, MD
Participating EMS Services: Maryland Institute for Emergency Medical Services Systems (MIEMSS), Baltimore City EMS
Footnotes
The authors report no conflicts of interest.
Meetings: This work has not been presented at a scientific meeting.
Author Contributions: JM and RS conceived the study; JM, EJ, KD, DWS, EZ, and RS designed the trial; RS obtained funding; EJ, KD, DWS, AP, NS, and EZ managed sample collection and processing. JM drafted the manuscript and all authors contributed significantly to its revision. JM takes responsibility for the paper as a whole.
References
- 1.Alldredge BK, Gelb AM, Isaacs SM, et al. A comparison of lorazepam, diazepam, and placebo for the treatment of out-of-hospital status epilepticus. N Engl J Med. 2001;345:631–7. doi: 10.1056/NEJMoa002141. [DOI] [PubMed] [Google Scholar]
- 2.Silbergleit R, Durkalski V, Lowenstein D, et al. Intramuscular versus intravenous therapy for prehospital status epilepticus. N Engl J Med. 2012;366:591–600. doi: 10.1056/NEJMoa1107494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Henry JC, Holloway R. Review: Lorazepam provides the best control for status epilepticus. ACP J Club. 2006;144(2):35. [PubMed] [Google Scholar]
- 4.Gottwald MD, Akers LC, Liu PK, et al. Prehospital stability of diazepam and lorazepam. Am J Emerg Med. 1999;17:333–7. doi: 10.1016/s0735-6757(99)90079-7. [DOI] [PubMed] [Google Scholar]
- 5.McMullan J, Sasson C, Pancioli A, Silbergleit R. Midazolam versus diazepam for the treatment of status epilepticus in children and young adults: a meta-analysis. Acad Emerg Med. 2010;17:575–82. doi: 10.1111/j.1553-2712.2010.00751.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bebin M, Bleck TP. New anticonvulsant drugs. Focus on flunarizine, fosphenytoin, midazolam and stiripentol. Drugs. 1994;48:153–71. doi: 10.2165/00003495-199448020-00003. [DOI] [PubMed] [Google Scholar]
- 7.USP. 23NF 18. United States Pharmacopeial Convention, Inc; Rockville, MD: 1995. p. 11. [Google Scholar]
- 8.Brown LH, Krumperman K, Fullagar CJ. Out-of-hospital medication storage temperatures: a review of the literature and directions for the future. Prehosp Emerg Care. 2004;8:200–6. doi: 10.1016/j.prehos.2003.12.017. [DOI] [PubMed] [Google Scholar]
- 9.USP Pharmacists' Pharmacopeia. United States Pharmacopeial Convention, Inc; Rockville, MD: 2005. p. v. [Google Scholar]
- 10. [Accessed March 24, 2012];Weather Underground. Available at: www.Weather.Underground.com.
- 11.Sokal RR, Rohlf FJ. Biometry; The Principles and Practice of Statistics in Biological Research. 3rd. San Francisco, CA: W. H. Freeman; 1995. [Google Scholar]
- 12.Gill MA, Kislik AZ, Gore L, Chandna A. Stability of advanced life support drugs in the field. Am J Health Syst Pharm. 2004;61:597–602. doi: 10.1093/ajhp/61.6.597. [DOI] [PubMed] [Google Scholar]