Introduction
Red blood cell (RBC) storage in the blood bank is associated with a sequence of biochemical, metabolic and mechanical changes leading to the progressive loss of cell viability and impairment of RBC survival upon transfusion into the recipient1–6. In detail, in erythrocytes stored for long periods, progressive haemolysis, alterations of permeability and flexibility, along with increased antigenicity are accompanied by an exacerbation of oxidative stress parameters, targeting both the lipid fraction (resulting in the accumulation of malonyldialdehyde and isoprostanes)3 and the protein fraction (with increases in the levels of carbonylated species, protein fragmentation and non-enzymatically glycated haemoglobin)3,5–11. The storage-dependent accumulation of reactive oxygen species (ROS) is mainly related to the progressive loss of metabolic modulation3,12, resulting in the impairment of anti-oxidant defences6. Indeed, in humans, each day about 3% of the body’s haemoglobin undergoes spontaneous autoxidation to methaemoglobin because of the high concentrations of iron in RBC6, whereby Fenton and Haber-Weiss reactions ultimately promote the accumulation of superoxyl and hydroxyl radicals, the latter being considered as the primary mechanism of injury in stored RBC6.
It is worth noting that exacerbated vesiculation during the storage of packed red cells can also be regarded as a self-protective mechanism to eliminate irreversibly altered components, such as carbonylated proteins13. In this view, preventing the accumulation of oxidative stress has been long been regarded as a viable strategy to improve the quality of stored RBC. Over the years, several approaches have been proposed to achieve this aim: (i) supplementation of metal chelators, such as deferoxamine, diethylenetriaminepentaacetic acid or ethylenediaminetetraacetic acid, which bind iron released from ageing erythrocytes, thus protecting RBC from the Fenton and Haber-Weiss reactions14; (ii) oxygen depletion15, to remove the main substrate of ROS-generating reactions and, in parallel, to improve the preservation of high energy phosphate compounds (adenosine triphosphate and diphosphoglycerate), at the expense of anti-oxidant defences16, by relying on the oxygen-dependent modulation of metabolic fluxes according to the model proposed by the groups of Low and Giardina17,18; (iii) supplementation of anti-oxidants in the additive solutions, using compounds containing thiol groups (glutathione loading)19 and vitamins (especially C in the form of ascorbate/dehydroascorbate and vitamin E)20–24.
This last strategy has attracted a great deal of interest during recent decades, especially in the light of the central role of glutathione (GSH) in intracellular anti-oxidant systems. Indeed, glutathione is the substrate for three key RBC anti-oxidant enzymes: glutathione peroxidase, glutathione reductase and glutathione S-transferase. Glutathione peroxidase-catalysed reactions inactivate H2O2 to neutral substances, water and oxidized glutathione (GSSG). Therefore, ROS are not generated in glutathione reactions, as is the case for superoxide dismutase (SOD)-catalysed reactions, from which it can be inferred that glutathione-related enzymatic mechanisms, rather than other enzymatic mechanisms, are primarily committed to protection against the auto-oxidation of erythrocytes25.
Oxidized glutathione is constantly being reduced by glutathione reductase through its utilization of NADPH, which is mainly generated via the pentose phosphate pathway. However, it has been recently confirmed that the progressive loss of metabolic modulation over storage proportionally impairs the capacity of RBC to replenish NADPH reservoirs via the pentose phosphate pathway, especially after the second week of storage2,12. In the light of this, alternative strategies could be pursued:
- to preserve GSH levels by promoting de novo synthesis of this tripeptide by fuelling a key limiting substrate precursor, cysteine, in the form of N-acetylcysteine (NAC)26;
or
Recently, we performed an extensive mass spectrometry-based metabolomics analysis to understand whether vitamin C and NAC supplementation improved storage quality, especially in terms of anti-oxidant potential and glutathione homeostasis and energy metabolism (Pallotta et al., unpublished data). Encouraging results suggested that loading of anti-oxidants boosted GSH levels and helped to prevent malondialdehyde accumulation and reduce haemolysis. However, we could not conclude whether anti-oxidants also preserved RBC morphology better during storage. In the light of the foregoing considerations, we investigated whether supplementation of ascorbate and NAC in the SAGM additive solution helped to preserve RBC morphology better throughout a 42-day storage period.
Materials and methods
Sample collection
Red blood cell units were drawn from healthy donor volunteers in accordance with the policy of the Italian National Blood Centre guidelines (Blood Transfusion Service for donated blood) and after informed consent in conformity with the declaration of Helsinki. We studied RBC units collected from 10 healthy male donor volunteers (age 39.4±7.5 [mean±SD] years). Leucofiltered (log4) packed red cell units were stored under standard conditions at 4 °C for up to 42 days (i) in the presence of CDP-SAGM, or (ii) in CPD-SAGM with the addition of ascorbic acid (0.23 mM-Sigma Aldrich, Milan, Italy) and NAC (0.5 mM-Sigma Aldrich). Dosing experiments for ascorbic acid and NAC were performed to minimise haemolysis at the end of the storage period. Sterility was assessed throughout the whole storage period.
Samples were removed aseptically for the analyses on a weekly basis. Samples for metabolomics studies were collected at 0, 7, 21, 28 and 42 days of storage.
Scanning electron microscopy
Scanning electron microscopy (SEM) studies of the control and treated RBC were performed using a JEOL JSM 5200 electron microscope on days 0, 28 and 42. The samples were prepared and the images analysed as previously reported4. SEM images were assembled with Photoshop CS 5.1 (Adobe Systems, Mountain View, CA, USA).
Results and discussion
Electron microscopy analyses evidenced substantial improvements in red blood cell morphology only in the first 28 days of storage
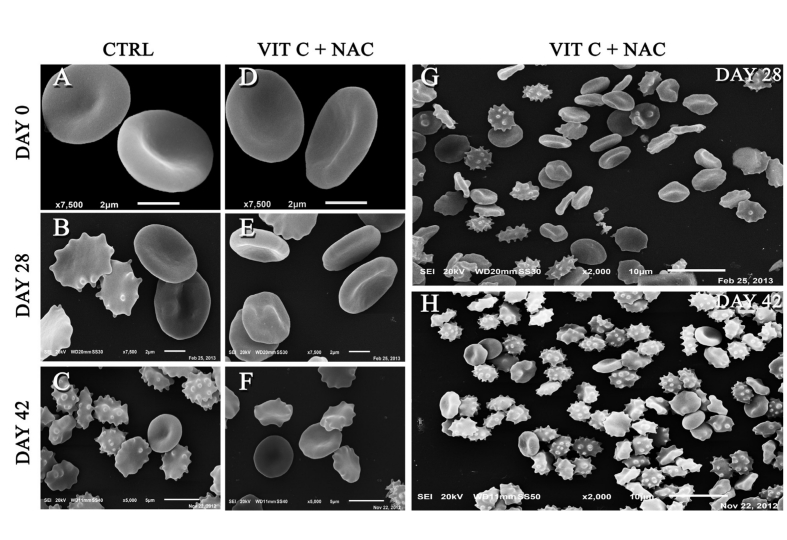
Storage in the presence of vitamin C and NAC improved the morphology score within the first 4 weeks of storage (Table I). However, supplementation with vitamin C and NAC did not produce any significant improvements at the end of the storage period (Table I). Indeed, at 42 days, the percentage of discocytes in supplemented units was not significantly higher than that in untreated controls (24.2%±2.1% vs 21.8%±1.6%; Table I), although the percentage of irreversibly altered RBC (including sphero-echinocytes, spherostomatocytes, spherocytes and degenerated shapes) was lower than in controls (29.5%±3.6% vs 34.6%±3.2%).
Table I.
SEM erythrocyte shape classification
| Storage day | Discocyte (%) | Reversibly* changed RBC (%) (echinocyte and stomatocyte shape) |
Irreversibly* changed RBC (%) (sphero-echinocyte, spherostomatocyte, spherocyte, valocyte, and degenerated shapes) |
|---|---|---|---|
| 0 | 77.3±2.9 | 18.4±4.6 | 4.3±3.1 |
| 28 Control |
46.1±3.8 | 36.2±1.4 | 17.7±2.6 |
| 28 Vitamin C+NAC |
51.8±1.3** | 32.6±2.9** | 15.5±1.8 |
| 42 Control |
21.8±1.6 | 43.6±2.7 | 34.6±3.2 |
| 42 Vitamin C+NAC |
24.2±2.1 | 46.3±1.9 | 29.5±3.6 |
Figure 1 shows a mosaic of SEM images, including control RBC stored in CPD-SAGM at days 0, 28 and 42 (Figure 1A, B and C, respectively), and RBC supplemented with vitamin C and NAC at the same times (Figure 1D, E and F, respectively). While the majority of red blood cells in supplemented units were still discocytes at 28 days (51.8%±1.3%; Table I, Figure 1.G), unaltered discocytes represented a minority of the RBC population and perfectly disc-shaped phenotypes were almost totally absent at the end of the storage period (Figure 1H).
Figure 1.
Scanning electron micrograph of control red blood cells (day 0 - A; day 28 - B; day 42 - C), and vitamin C+NAC-supplemented red blood cells (day 0 - D; day 28 - E; day 42 - F). In E and F, 2,000× magnification of vitamin C+NAC-supplemented red blood cells at 28 and 42 days, respectively. Scale bars and magnification values are reported in each panel. Panels were assembled with Photoshop CS 5.1 (Adobe Systems, Mountain View, CA, USA).
A direct comparison of these results against those of our recent study on changes in RBC morphology arising upon deoxygenation29 suggests that oxygen removal might preserve RBC morphology better than the hereby-discussed supplementation of anti-oxidants.
It is worth recalling that an increase in intracellular calcium directly induces or indirectly promotes a cascade of molecular and cellular events such as loss of deformability, and echinocytosis30, besides protein degradation/cross-linking, recognition signalling and, ulitmately, eryptosis31. This is particularly true in units that have not been leucofiltered31, which exacerbates oxidative stress. Indeed, oxidative stress is increasingly emerging as an additional trigger to calcium-dependent morphological lesions of RBC32, which likely results from the close cross-talk between energy and redox metabolism in RBC, especially in the blood bank2. Future investigations might, therefore, be designed to test whether metabolic (Pallotta et al., unpublished data) and morphological (present study) changes observed in RBC stored with anti-oxidant supplementats (vitamin C and NAC) might be correlated with alterations in intracellular calcium reservoirs.
Conclusion
Although oxidative stress represents a critical challenge for RBC under blood bank storage conditions, boosting redox metabolism alone does not seem to be sufficient to completely prevent and cope with the lesions targeting membrane morphology. On the other hand, when compared to untreated controls, supplementation of anti-oxidants helped to preserve RBC morphology for a broader shelf-life window (the first 4 weeks of storage), which suggests that supplementation of anti-oxidants might be a viable strategy to improve storage quality, rather than to extend storage duration of erythrocyte concentrates.
Future investigations might address the effects of the combination of deoxygenation and anti-oxidant supplementation strategies on storage quality of erythrocyte concentrates.
Acknowledgements
Valeria Pallotta, Federica Gevi, Angelo D’Alessandro and Lello Zolla are supported by funds from the National Blood Centre, National Institute of Health (Centro Nazionale Sangue [CNS], Istituto Superiore Sanità, Rome, Italy). Angelo D’Alessandro was supported by mobility studentship funds from the Interuniversity Consortium for Biotechnologies (CIB). The authors are grateful to Dr. Gabriella Gambellini for technical assistance with SEM analyses. The authors are grateful to Dr. Francesca Caravello for her technical assistance with the study design, within the framework of her M.Sc. internship training.
Footnotes
The Authors declare no conflicts of interest.
References
- 1.D’Alessandro A, Liumbruno G, Grazzini G, Zolla L. Red blood cell storage: the story so far. Blood Transfus. 2010;8:82–8. doi: 10.2450/2009.0122-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gevi F, D’Alessandro A, Rinalducci S, Zolla L. Alterations of red blood cell metabolome during cold liquid storage of erythrocyte concentrates in CPD-SAGM. J Proteomics. 2012;76:10–27. doi: 10.1016/j.jprot.2012.03.012. [DOI] [PubMed] [Google Scholar]
- 3.D’Alessandro A, D’Amici GM, Vaglio S, Zolla L. Time-course investigation of SAGM-stored erythrocyte concentrates: from metabolism to proteomics. Haematologica. 2012;97:107–15. doi: 10.3324/haematol.2011.051789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blasi B, D’Alessandro A, Ramundo N, Zolla L. Red blood cell storage and cell morphology. Transfus Med. 2012;22:90–6. doi: 10.1111/j.1365-3148.2012.01139.x. [DOI] [PubMed] [Google Scholar]
- 5.Lachant NA, Noble NA, Myrhe BA, Tanaka KR. Antioxidant metabolism during blood storage and its relationship to posttransfusion red cell survival. Am J Hematol. 1984;17:237–49. doi: 10.1002/ajh.2830170304. [DOI] [PubMed] [Google Scholar]
- 6.Jóźwik M, Jóźwik M, Jóźwik M, et al. Antioxidant defence of red blood cells and plasma in stored human blood. Clin Chim Acta. 1997;267:129–42. doi: 10.1016/s0009-8981(97)00148-4. [DOI] [PubMed] [Google Scholar]
- 7.Ogunro PS, Ogungbamigbe TO, Muhibi MA. The influence of storage period on the antioxidants level of red blood cells and the plasma before transfusion. Afr J Med Med Sci. 2010;39:99–104. [PubMed] [Google Scholar]
- 8.Noble NA, Tanaka KR, Myhre BA, Johnson DE. Red cell enzyme activity during blood storage and reactivation of phosphofructokinase. Am J Hematol. 1982;13:1–8. doi: 10.1002/ajh.2830130102. [DOI] [PubMed] [Google Scholar]
- 9.D’Alessandro A, Mirasole C, Zolla L. Haemoglobin glycation (Hb1Ac) increases during red blood cell storage: a MALDI-TOF mass-spectrometry-based investigation. Vox Sang. 2013;105:177–80. doi: 10.1111/vox.12029. [DOI] [PubMed] [Google Scholar]
- 10.Kriebardis AG, Antonelou MH, Stamoulis KE, et al. Progressive oxidation of cytoskeletal proteins and accumulation of denatured hemoglobin in stored red cells. J Cell Mol Med. 2007;11:148–55. doi: 10.1111/j.1582-4934.2007.00008.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Delobel J, Prudent M, Rubin O, et al. Subcellular fractionation of stored red blood cells reveals a compartment-based protein carbonylation evolution. J Proteomics. 2012;76:181–93. doi: 10.1016/j.jprot.2012.05.004. [DOI] [PubMed] [Google Scholar]
- 12.Messana I, Ferroni L, Misiti F, et al. Blood bank conditions and RBCs: the progressive loss of metabolic modulation. Transfusion. 2000;40:353–60. doi: 10.1046/j.1537-2995.2000.40030353.x. [DOI] [PubMed] [Google Scholar]
- 13.Willekens FL, Werre JM, Groenen-Döpp YA, et al. Erythrocyte vesiculation: a self-protective mechanism? Br J Haematol. 2008;141:549–56. doi: 10.1111/j.1365-2141.2008.07055.x. [DOI] [PubMed] [Google Scholar]
- 14.Knight A, Voorhees RP, Martin L. The effect of metal chelators on lipid peroxidation in stored erythrocytes. Ann Clin Lab Sci. 1992;22:207–13. [PubMed] [Google Scholar]
- 15.Yoshida T, Shevkoplyas SS. Anaerobic storage of red blood cells. Blood Transfus. 2010;8:220–36. doi: 10.2450/2010.0022-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.D’Alessandro A, Gevi F, Zolla L. Red blood cell metabolism under prolonged anaerobic storage. Mol Biosystems. 2013;9:1196–209. doi: 10.1039/c3mb25575a. [DOI] [PubMed] [Google Scholar]
- 17.Lewis IA, Campanella ME, Markley JL, Low PS. Role of band 3 in regulating metabolic flux of red blood cells. Proc Natl Acad Sci USA. 2009;106:18515–20. doi: 10.1073/pnas.0905999106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Castagnola M, Messana I, Sanna MT, Giardina B. Oxygen-linked modulation of erythrocyte metabolism: state of the art. Blood Transfus. 2010;8(Suppl 3):s53–8. doi: 10.2450/2010.009S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dumaswala UJ, Wilson MJ, Wu YL, et al. Glutathione loading prevents free radical injury in red blood cells after storage. Free Radic Res. 2000;33:517–29. doi: 10.1080/10715760000301061. [DOI] [PubMed] [Google Scholar]
- 20.Knight JA, Blaylock RC, Searles DA. The effect of vitamins C and E on lipid peroxidation in stored erythrocytes. Ann Clin Lab Sci. 1993;23:51–6. [PubMed] [Google Scholar]
- 21.Dawson RB, Hershey RT, Myers CS, Miller RM. Blood preservation 35. Red cell 2,3-DPG and ATP maintained by DHA-ascorbate-phosphate. Transfusion. 1981;21:219–23. doi: 10.1046/j.1537-2995.1981.21281178161.x. [DOI] [PubMed] [Google Scholar]
- 22.Dawson RB, Hershey RT, Myers CS, Eaton JW. Blood preservation XLIV. 2,3-DPG maintenance by dehydroascorbate better than D-ascorbic acid. Transfusion. 1980;20:321–3. doi: 10.1046/j.1537-2995.1980.20380214899.x. [DOI] [PubMed] [Google Scholar]
- 23.Magnusardottir AR, Skuladottir GV. Effects of storage time and added antioxidant on fatty acid composition of red blood cells at −20 degrees C. Lipids. 2006;41:401–4. doi: 10.1007/s11745-006-5112-8. [DOI] [PubMed] [Google Scholar]
- 24.Ma EP, Liu XZ, Han Y, et al. New tactics of human red blood cells stored at 4 degrees C-protective effect of antioxidant solution on red blood cells damage. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2002;10:153–5. Abstract. [PubMed] [Google Scholar]
- 25.Raftos JE, Whillier S, Kuchel PW. Glutathione synthesis and turnover in the human erythrocyte: alignment of a model based on detailed enzyme kinetics with experimental data. J Biol Chem. 2010;285:23557–67. doi: 10.1074/jbc.M109.067017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Whillier S, Raftos JE, Chapman B, Kuchel PW. Role of N-acetylcysteine and cystine in glutathione synthesis in human erythrocytes. Redox Rep. 2009;14:115–24. doi: 10.1179/135100009X392539. [DOI] [PubMed] [Google Scholar]
- 27.May JM. Ascorbate function and metabolism in the human erythrocyte. Front Biosci. 1998;3:d1–10. doi: 10.2741/a262. [DOI] [PubMed] [Google Scholar]
- 28.Rizvi SI, Pandey KB, Jha R, Maurya PK. Ascorbate recycling by erythrocytes during aging in humans. Rejuvenation Res. 2009;12:3–6. doi: 10.1089/rej.2008.0787. [DOI] [PubMed] [Google Scholar]
- 29.Zolla L, D’Alessandro A. An efficient apparatus for rapid deoxygenation of erythrocyte concentrates for alternative banking strategies. J Blood Transfus. 2013;2013:896537. doi: 10.1155/2013/896537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reinhart WH, Chien S. Echinocyte-stomatocyte transformation and shape control of human red blood cells: morphological aspects. Am J Hematol. 1987;24:1–14. doi: 10.1002/ajh.2830240102. [DOI] [PubMed] [Google Scholar]
- 31.Antonelou MH, Tzounakas VL, Velentzas AD, et al. Effects of pre-storage leukoreduction on stored red blood cells signaling: a time-course evaluation from shape to proteome. J Proteomics. 2012;76:220–38. doi: 10.1016/j.jprot.2012.06.032. [DOI] [PubMed] [Google Scholar]
- 32.Antonelou MH, Kriebardis AG, Velentzas AD, et al. Oxidative stress-associated shape transformation and membrane proteome remodeling in erythrocytes of end stage renal disease patients on hemodialysis. J Proteomics. 2011;74:2441–52. doi: 10.1016/j.jprot.2011.04.009. [DOI] [PubMed] [Google Scholar]