Fig. 8.
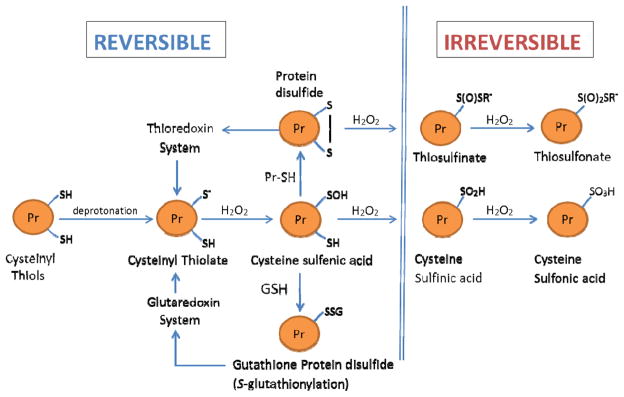
Redox stress: Schema of the modifications of cysteinyl thiols of redox-sensitive proteins in response to elevation in H2O2 fluxes. Under physiological conditions, protein cysteinyl thiols, involved in cell signaling, exist as deprotonated thiolate anions (Pr-S−), which have enhanced reactivity with H2O2. Oxidation of Pr-S− by H2O2 results in the formation of cysteine sulfenic acid (Pr-SOH), which is a relatively unstable molecule. In turn, Pr-SOH may react with GSH to form a mixed protein disulfide (Pr-SSG) or alternatively it may form a disulfide bridge with a Pr-SH to form Pr-S-S-Pr. Such oxidations of Pr-S− are reversed by the glutaredoxin and thioredoxin systems, respectively. At relatively high intra-cellular concentrations of H2O2, Pr-SOH may be further oxidized to cysteine sulfinic acid (Pr-SOOH) and cysteine sulfonic acid (Pr-SOOH), both of which are considered to be irreversible products. Similarly, protein disulfides Pr-S-S-R’ may undergo further irreversible oxidations to thiosulfinate or thiosulfonate, as described by Brandes et al. [221].
