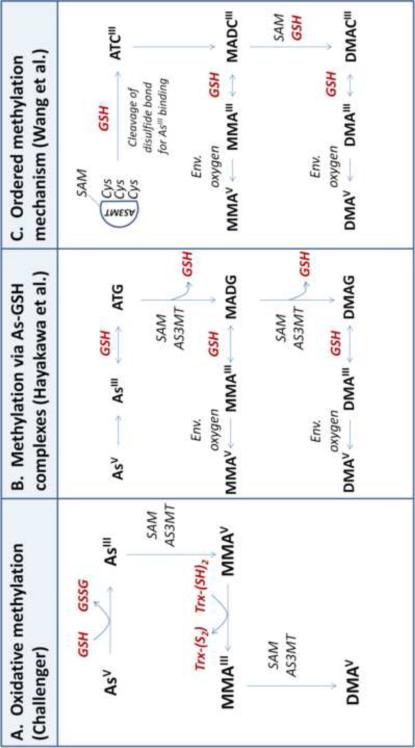
Figure 1. Involvment of glutathione in arsenic methylation mechanisms.
Inorganic arsenic (InAs) is metabolized in the liver via a series of methylation reactions, resulting in the formation of monomethylarsonic acid (MMA) and dimethylarsinic acid (DMA). Glutathione (GSH), while not absolutely required for As methylation, can serve as the reductant necessary for AS3MT activity. Three distinct AS3MT-catalyzed methylation pathways have been proposed in the literature (A-C):
A. Oxidative methylation. In this pathway first proposed by Challenger [21, 22], AS3MT catalyzes the oxidative methylation of arsenite (AsIII) and methylarsonous acid (MMAIII) to methylarsonic acid (MMAV) and dimethylarsinic acid (DMAV), respectively. A reductant (such as GSH) is required for the reduction of the pentavalent arsenicals (AsV and MMAV) to their trivalent counterparts (AsIII and MMAIII).
B. Successive methylation via As-GSH complexes. Hayakawa et al. [23] proposes a pathway in which As-GSH complexes are substrates for AS3MT. Here, AsIII nonenzymatically complexes with GSH to generate arsenic triglutathione (ATG), which is methylated by AS3MT to form monomethylarsonic diglutathione (MADG). MADG can be further methylated to dimethylarsinic glutathione (DMAG). As-GSH complexes (ATG, MADG, and DMAG) are in equilibrium with trivalent arsenicals (AsIII, MMAIII, and DMAIII) based on the concentration of GSH. The trivalent methylated arsenicals (MMAIII and DMAIII) can be oxidized to pentavalent arsenicals (MMAV and DMAV) by environmental oxygen.
C. Ordered methylation mechanism. Wang et al. [24] propose a model in which a reductant (thiol or nonthiol) changes the conformation of AS3MT to influence enzyme activity. In this scheme, SAM binds to AS3MT; then, a reductant (such as GSH) cleaves a disulfide bond in the enzyme, which exposes active-site cysteine (Cys) residues for AsIII binding. AsIII binds the Cys residues, forming arsenic tricysteine (ATCIII), and the methyl group from SAM is transferred to ATCIII on the AS3MT enzyme, resulting in monomethylarsonic dicysteine (MADCIII). MADCIII can remain bound to AS3MT and be further methylated to dimethylarsinic cysteine (DAMCIII). MADCIII and DAMCIII can dissociate from AS3MT, forming MMAIII and DMAIII, respectively. Trivalent species are then oxidized to pentavalent arsenicals by environmental oxygen.