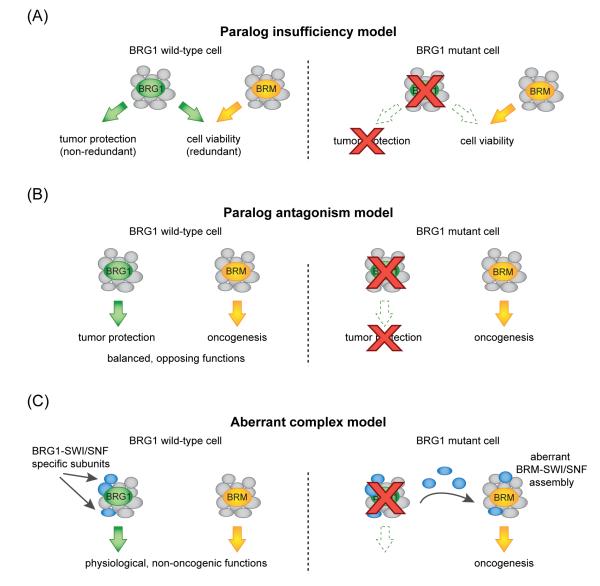
Figure 2.
Hypothetical models for the function of residual BRM-SWI/SNF complexes in BRG1-mutant cancers. A) Paralog insufficiency model. In the cancer cell-of-origin, BRG1 and BRM perform redundant functions in supporting cell viability while BRG1 performs a non-redundant tumor suppressor function. Loss of BRG1 would lead to tumorigenic effects while simultaneously rendering BRM the sole ATPase subunit responsible for supporting tumor cell viability. B) Paralog antagonism model. In the cancer cell-of-origin, BRG1 performs a specific function in tumor protection while BRM promotes oncogenesis, resulting in a balanced state of SWI/SNF functions. Loss of BRG1 would result in unopposed BRM-driven proliferation and tumorigenesis. C) Aberrant complex model. Loss of BRG1 would release specific subunits of its dedicated protein complex, which would form aberrant associations with BRM that deregulate cancer-relevant transcriptional programs.