Abstract
Background
Disease-modifying anti-rheumatic drugs (DMARDs) are the standard of care for rheumatoid arthritis (RA), however studies have found that many patients do not receive them. We examined predictors of starting and stopping DMARDs among a longitudinal cohort of patients with RA.
Methods
Study participants came from a cohort of RA patients recruited from a random sample of rheumatologists’ practices in Northern California. We examined patterns and predictors of stopping and starting non-biologic and biologic DMARDs during 1982–2009 based on annual questionnaires. Stopping was defined as stopping all DMARDs and starting was defined as transitioning from no DMARDs to any DMARDs across two consecutive years.
Results
The analysis of starting DMARDs included 471 subjects with 1,974 pairs of years with no DMARD use in the first of two consecutive years. From this population, subjects started DMARD use by year two in 313 of the pairs (15.9%). The analysis of stopping DMARDs included 1,026 subjects with 7,595 pairs of years with DMARD use in the first of two consecutive years; in 423 (5.6%), subjects stopped DMARD use by year two. In models that adjusted for RA-related factors, sociodemographics and comorbidities, significant predictors of starting DMARDs included younger age, Hispanic ethnicity, shorter disease duration, and the use of oral glucocorticoids. In separate adjusted models, predictors of stopping DMARDs included Hispanic ethnicity, and low income, while younger age was associated with a reduced risk of stopping.
Conclusion
Efforts to improve DMARD use should focus on patient age, ethnicity, and income, and RA-related factors.
INTRODUCTION
Since 2002, the American College of Rheumatology has recommended the use of disease-modifying anti-rheumatic drugs (DMARDs) for all patients with rheumatoid arthritis (RA). (1) However, many RA patients appear not to be using DMARDs. (2–4) Some of this variation is likely due to whether RA is defined using standard classification criteria versus other methods. Many prior studies investigating predictors of DMARD use have relied on insurance databases that use diagnostic codes (2, 3, 5, 6) or population-based surveys. (4) The positive predictive value associated with defining an RA cohort solely on diagnosis codes is lower than with definitions that include use of DMARDs (7), but including DMARD dispensing in the cohort definition will bias towards over-sampling subjects more likely to be current DMARD users. Thus, for the purposes of studying predictors of DMARD use, there is value to studying a cohort of patients with definite RA as diagnosed by a rheumatologist in which some patients consistently use DMARDs and others do not.
Prior studies have found that DMARD use is related to RA-related and unrelated factors. Older age has been consistently related to reduced DMARD use. (2–4) An increased number of comorbidities and lower annual incomes have also been associated with reduced DMARD use. (2, 4) We recently published data from the National Ambulatory Medical Care Survey showing that black race was associated with a reduced probability of receipt of DMARDs. (4) The strongest and most consistent correlate of DMARD use is a rheumatology visit. (2, 4, 8)
The UCSF RA Panel (the “Panel”) is a longitudinal cohort of patients with RA recruited from rheumatologists’ offices in Northern California. (9) Participants were surveyed annually since 1982, with additional recruitment during the last 30 years. Because of the long-term nature of this cohort diagnosed with RA by rheumatologists, the Panel provides a distinctive opportunity to study DMARD use patterns and predictors in a sample with more clinically rich data than what might be found in an insurance claims cohort or a national survey. Specifically, we focused on patterns of DMARD stopping and starting, examining three types of predictors: RA-related, sociodemographic, and comorbid conditions. We hypothesized that RA-related as well as other types of predictors would each predict use of DMARDs.
METHODS
Study Design and Cohort
This study is based on data (1982–2009) from the University of California, San Francisco (UCSF) RA Panel study which includes 1,507 people with RA from the practices of 50 randomly sampled rheumatologists in Northern California. The initial Panel included 882 subjects enrolled between 1982 and 1983; further recruitment occurred in 1989, 1995, 1999 and 2003. Practices represented community-based rheumatologists, some of whom were in solo or group fee-for-service while others were in health maintenance organizations. Other details about the structure of the Panel and the validity of its measures are summarized elsewhere. (9–11) Patients provided written informed consent at the time of entering the Panel.
The principal data collection method for the RA Panel study is an annual, structured telephone interview conducted by a trained survey worker. These surveys collect basic demographic information, signs and symptoms of RA, number of comorbid conditions, RA treatment, physical and psychological health status, functional status, health care utilization information, and characteristics of health insurance plans. The RA treatment question (the outcome of interest for the current analyses) asked patients to answer the following item for each RA treatment separately: “Have you taken [DMARD] for at least 1 month during the past year?” Answering yes to any of the DMARDs qualified a subject as using any DMARD. Subjects answering no to all of the DMARD use questions were classified as non-users.
To assess the predictors of starting or stopping a DMARD, we created two separate cohorts of eligible subjects from the UCSF Panel Survey. For the analysis of DMARD starters, subjects were identified who were not using any DMARD at a given annual questionnaire. Similarly, for the analysis of DMARD stoppers, subjects were identified who reported using at least one DMARD during a given annual questionnaire. Thus, the same subject could contribute more than one observation; s/he could contribute to both the DMARD starter and stopper analysis during different years of follow-up.
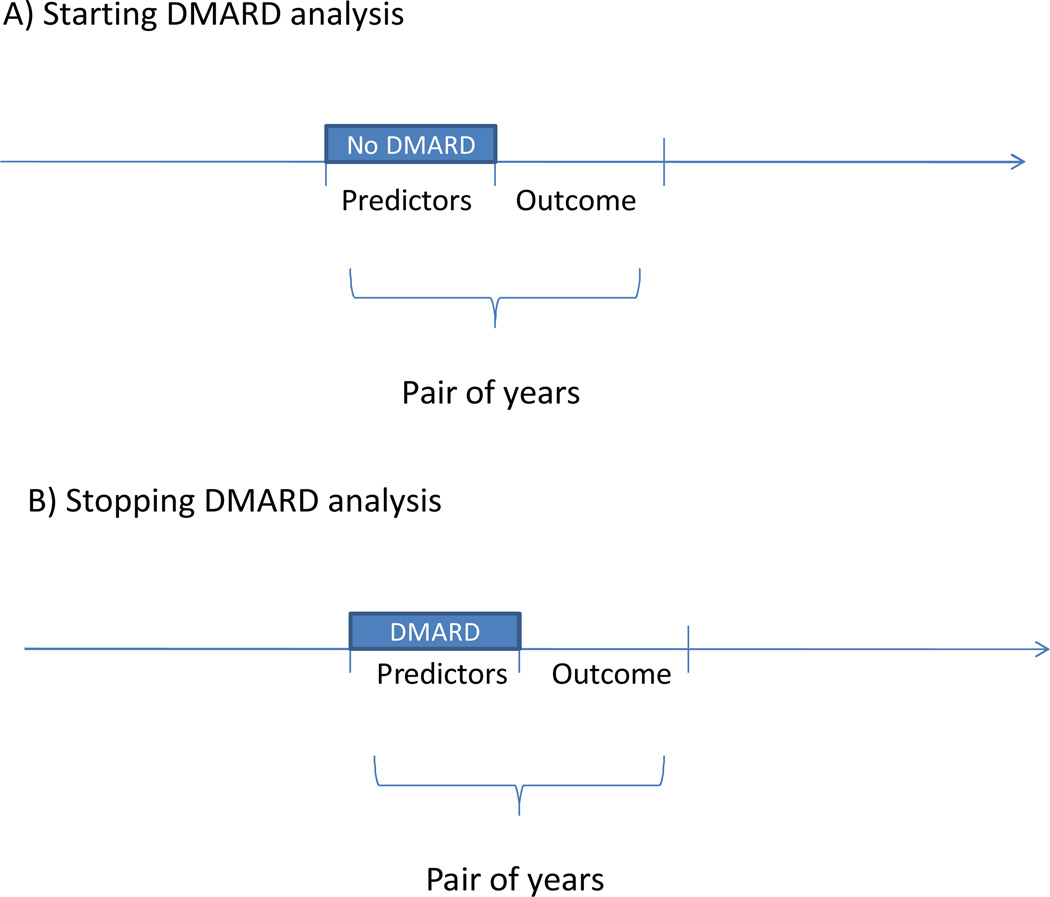
We examined pairs of years, assessing predictors in the first of the two year pair and DMARD outcomes in the second year (see Figure 1); DMARD starting was examined among patients taking no DMARDs in the first year and stopping among those taking at least one DMARD in the first year. We required subjects to have data for both years in a given pair, thus missing data issues were avoided. The study was approved by the UCSF and Brigham and Women’s Hospital’s Institutional Review Boards.
Figure 1.
This figure illustrates the study design in which we examined pairs of years during the several decades of follow-up. Thus, a given patient could be included as multiple observations in either the starting DMARD (panel A) or stopping DMARD analyses (panel B). All predictor variables were examined in year 1 and outcomes assessed in year 2.
DMARD Use Outcomes
DMARD stopping was only assessed among subjects who reported use of a DMARD in the first of two consecutive years. The goal of these analyses was examining patterns and predictors of starting and/or stopping DMARDs, not switching DMARDs. A subject qualified as stopping DMARDs if all such agents were discontinued, not if s/he stopped one of several DMARDs being used. Thus, stopping was determined based on subjects being users of at least one DMARD during year one and of no DMARDs in year two. In a parallel fashion, a subject must have been using no DMARDs in the first of the pair of years to be assessed for the DMARD starting analysis. We then examined if any DMARD was begun in the second of two years.
This study covered several decades and many possible DMARDs, including the following non-biologic DMARDs: azathioprine, cyclophosphamide, cyclosporine, d-penicillamine, oral or injectable gold compounds, hydroxychloroquine, leflunomide, methotrexate and sulfasalazine. For biologic DMARDs, we assessed use of abatacept, adalimumab, anakinra, etanercept, infliximab, rituximab, and tocilizumab; but, no participants reported use of rituximab or tocilizumab.
Predictors of Starting and Stopping
We examined potential predictors of stopping and/or starting DMARDs during the first of the two years. One focus of this research was to assess the contribution of different types of variables. The variable types chosen follow a commonly used model of health care services utilization proposed by Aday and Anderson that considers enabling, predisposing, and patient need. (12) Thus, predictors were categorized into several groups: sociodemographic variables (age, gender, race, ethnicity, health insurance status and type, educational attainment, annual income, and marital status); RA-related variables (disease duration, health assessment questionnaire, (13) self-assessed number of tender joints, self-assessed number of swollen joints, and use of oral glucocorticoids); and comorbid conditions (a current comorbidity count, a depression scale, (14) the use of non-DMARD medications, and acute care hospitalizations). Finally, visits to a rheumatologist were considered as a covariate. This variable was not included in any of the above categories. We grouped the number of rheumatology visits over the prior year into none, 1–6 visits and more than 6 visits based on the distribution of the responses.
Statistical Analyses
After forming the starter and stopper cohorts, we defined the characteristics of subjects in each group. These were defined for each potential starting and stopping period (allowing one subject to contribute multiple periods), as well as by subject. Trends in starting and stopping DMARDs were described over the study period, calculating percentages on an annual basis and identifying which DMARDs were started and stopped. We examined predictors of starting and stopping DMARDs separately in generalized linear mixed models that account for the non-independence of records from multiple pairs of years for an individual subject; the covariance matrix utilized a compound symmetry correlation structure according to the Akaike Information Criterion (AIC) and Bayesian Information Criterion (BIC). Models were fit for each category of potential predictor (sociodemographic, RA-related, and comorbidities) separately and then including all potential predictors in fully adjusted models. We assessed the model fit using the c-statistic calculated in logistic regression models. The c-statistics were reported across the categories of potential predictors – sociodemographics, RA-related, and comorbidities.
The above models were fit without inclusion of rheumatology visits as a potential predictor. Since this variable has been a strong and consistent predictor of DMARD use patterns, (2, 4) we examined other types of variables in the primary analysis. In a secondary analysis, rheumatology visits were added to the final multivariable models.
All analyses were conducted using SAS Statistical Software, version 9.2 (Cary, NC).
RESULTS
From the UCSF RA Panel, we identified 1,687 subjects with 1,974 eligible periods without any DMARD use in the first year; these periods were examined as the potential DMARD starters. Similarly, we identified 1,874 subjects with 7,595 eligible periods with DMARD use in the first year; these periods were examined as the potential DMARD stoppers.
The characteristics of the UCSF RA Panel participants at each of the starting and stopping periods are shown in Table 1. (Baseline characteristics of participants without including every period, but only the first one, are shown in Supplemental Table 1.) The mean age of the Panel at the time of the starting and stopping periods was 60 years with 81% female. The majority of subjects were non-Hispanic White and most had greater than a high school education. Few subjects lacked insurance and most were in fee for service insurance programs. Mean RA disease duration was 18 years and the mean HAQ was 1.1. About half of subjects had no comorbid conditions reported in the current questionnaire. About half of all subjects reported using oral glucocorticoids.
Table 1.
Baseline characteristics of the total UCSF RA Panel and the stopping and starting periods
| Total Periods (n = 9,569) |
Stopper Periods (n = 7,595) |
Starter Periods (n = 1,974) |
|
|---|---|---|---|
| N (%) unless noted | |||
| Sociodemographics | |||
| Age, mean (SD), years | 60 ± 13 | 60 ± 13 | 63 ± 14 |
| Female | 7706 (81) | 6156 (81) | 1550 (79) |
| Ethnicity | |||
| Hispanic | 821 (9) | 661 (9) | 160 (8) |
| African American | 253 (3) | 147 (2) | 106 (5) |
| Asian and Pacific Islanders | 485 (5) | 363 (5) | 122 (6) |
| Other | 181 (2) | 150 (2) | 31 (2) |
| Non-Hispanic White | 7829 (82) | 6274 (83) | 1555 (79) |
| Marital status | |||
| Unmarried/Divorced | 3368 (35) | 2571 (34) | |
| Married/Partner | 6201 (65) | 5024 (66) | 1177 (60) |
| Educational attainment | |||
| < High school | 1158 (12) | 835 (11) | 323 (16) |
| High school degree | 3009 (31) | 2397 (32) | 612 (31) |
| > High school | 5402 (56) | 4363 (57) | 1039 (53) |
| Annual household income | |||
| Lowest category | 1164 (12) | 826 (11) | 338 (17) |
| Low-middle category | 2642 (28) | 2027 (27) | 615 (31) |
| High-middle category | 4482 (47) | 3688 (49) | 794 (40) |
| Highest category | 1281 (13) | 1054 (14) | 227 (12) |
| Insurance Status | |||
| Primary Health Insurance | |||
| Private/Medicaid/Medicare | 9426 (99) | 7482 (99) | 1944 (98) |
| No Insurance | 143 (1) | 113 (1) | 30 (2) |
| Managed Care | |||
| Fee for service | 4031 (42) | 3083 (41) | 948 (48) |
| HMO/PPO | 5538 (58) | 4512 (59) | 1026 (52) |
| 1st year of observation, median (range) | 1988 (1986–2006) | 1989 (1986–2007) | 1993 (1986–2008) |
| Paired years of observation after 1999 | 3964 (41) | 3405 (45) | 559 (28) |
| RA Related Factors | |||
| Disease duration, mean ± SD | 18 ± 11 | 18 ± 11 | 20 ± 11 |
| HAQ, mean ± SD | 1.1 ± 0.7 | 1.1 ± 0.7 | 1.2 ± 0.8 |
| Number of painful joints, mean ± SD | 6.1 ± 4.5 | 6.0 ± 4.5 | 6.3 ± 4.8 |
| Number of swollen joints, mean ± SD | 3.6 ± 3.2 | 3.6 ± 3.2 | 3.6 ± 3.3 |
| Comorbidities | |||
| Geriatric depression scale > 7 | 1045 (11) | 788 (10) | 257 (13) |
| Comorbidities | |||
| None | 5277 (55) | 4190 (55) | 1087 (55) |
| One | 2937 (31) | 2370 (31) | 567 (29) |
| Two or more | 1355 (14) | 1035 (14) | 320 (16) |
| Oral glucocorticoid use | 4892 (51) | 4044 (53) | 848 (43) |
| Use non-DMARD meds for RA | 4483 (47) | 3923 (52) | 560 (28) |
| Hospitalized in prior 12 months | 2245 (23) | 1709 (23) | 536 (27) |
Notes: The same subject can contribute to both cohorts and multiple times based on how many eligible stopping and starting periods s/he experienced.
Abbreviations: DMARD, disease-modifying anti-rheumatic drug; HMO, health maintenance organization; PPO, preferred provider organization; HAQ, Health Assessment Questionnaire; RA, rheumatoid arthritis.
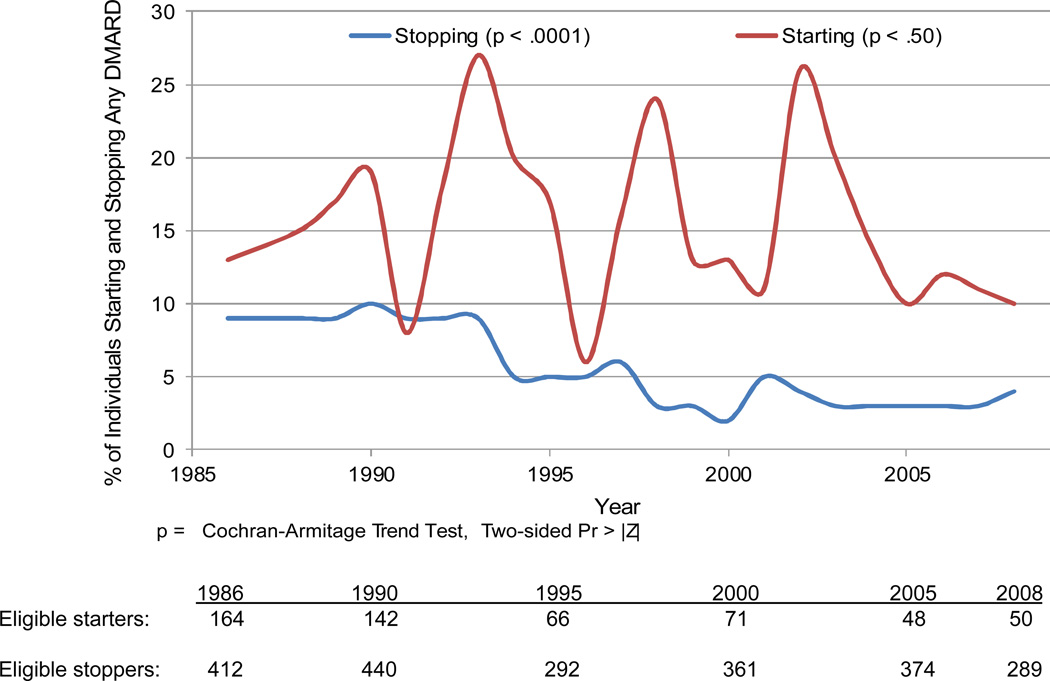
The analysis of starting DMARDs included 471 subjects with 1,974 pairs of years with no DMARD use in the first of two consecutive years. From this population, subjects started DMARD use by year two in 313 of the pairs (15.9%). The percentage of potential DMARD starters who started these agents varied across years with no significant temporal trend (p for trend 0.50) (see Figure 2). The analysis of stopping DMARDs included 1,026 subjects with 7,595 pairs of years with DMARD use in the first of two consecutive years; in 423 (5.6%), subjects stopped DMARD use by year two. The percentage of potential DMARD stoppers who did stop decreased steadily from 9% to 3% (p for trend < 0.001). Drugs that were started and stopped reflect typical DMARDs in use over the study period. The most commonly started and stopped DMARDs were methotrexate (28% of all started medications and 20% of all those stopped) and hydroxychloroquine (15% of both started and stopped medications) (see Supplemental Table 2).
Figure 2.
This figure illustrates the percentage of subjects starting and stopping DMARDs in a given year during follow-up. Below the figure, we indicate the potential starters and stoppers (denominators) for given selected years.
We examined predictors of starting and stopping DMARDs in separate adjusted regression models (see Table 2). The fully adjusted model demonstrated the following significant predictors of starting DMARDs: younger age (OR 1.30, 95% CI 1.13 – 1.50 per decade decrease, Hispanic ethnicity (OR 1.88, 95% CI 1.06 – 3.33), shorter disease duration (OR 1.11, 95% CI 1.01 – 1.22 per 5-year decrease), and the use of oral glucocorticoids (OR 1.91, 95% CI 1.36 – 2.66). In a separate fully adjusted model, significant predictors of stopping DMARDs included younger age (OR 0.88, 95% CI 0.80 – 0.98per decade decrease), Hispanic ethnicity (OR 1.52, 95% CI 1.02 – 2.30), and lowest annual income compared with highest (OR 1.83, 95% CI 1.13 – 2.96).
Table 2.
Fully adjusted generalized linear mixed regression models fit for starting and stopping DMARDs
| Models without adjustment for rheumatology visit |
Model adjusted for rheumatology visit | |||
|---|---|---|---|---|
| Starting DMARDs | Stopping DMARDs | Starting DMARDs | Stopping DMARDs | |
| Adjusted Odds Ratio (95% CI) | Adjusted Odds Ratio (95% CI) | |||
| Sociodemographic | ||||
| Age, per decade decrease | 1.30 (1.13 – 1.50) | 0.88 (0.80 – 0.98) | 1.28 (1.12 – 1.48) | 0.89 (0.80 – 0.99) |
| Gender, female | 1.37 (0.86 – 2.17) | 0.99 (0.72 – 1.35) | 1.35 (0.85 – 2.13) | 0.99 (0.72 – 1.35) |
| Race/ethnicity | ||||
| Hispanic | 1.88 (1.06 – 3.33) | 1.52 (1.02 – 2.30) | 1.65 (0.93 – 2.92) | 1.59 (1.06 – 2.38) |
| Black, non-Hispanic | 0.42 (0.17 – 1.04) | 1.39 (0.66 – 2.95) | 0.40 (0.16 – 0.99) | 1.32 (0.62 – 2.82) |
| Asian*, non-Hispanic | 0.71 (0.35 – 1.45) | 1.22 (0.69 – 2.95) | 0.69 (0.34 – 1.39) | 1.14 (0.65 – 2.01) |
| Other, non-Hispanic | 1.31 (0.33 – 5.29) | 0.56 (0.18 – 1.72) | 1.23 (0.31 – 4.85) | 0.57 (0.19 – 1.74) |
| Non-Hispanic White | 1.00 | 1.00 | 1.00 | 1.00 |
| Educational attainment | ||||
| Less than high school | 0.85 (0.50 – 1.45) | 0.94 (0.64 – 1.40) | 0.89 (0.52 – 1.51) | 0.91 (0.62 – 1.37) |
| High school only | 0.90 (0.60 – 1.34) | 1.03 (0.78 – 1.36) | 0.90 (0.61 – 1.34) | 1.04 (0.79 – 1.37) |
| Beyond high school | 1.00 | 1.00 | 1.00 | 1.00 |
| Annual household income | ||||
| Quartile 1 (lowest) | 0.80 (0.45 1.44) | 1.83 (1.13 – 2.96) | 0.83 (0.46 – 1.49) | 1.61 (1.04 – 2.49) |
| Quartile 2 | 0.80 (0.51 – 1.26) | 1.23 (0.81 – 1.85) | 0.83 ( 0.53 – 1.30) | 1.02 (0.75 – 1.45) |
| Quartile 3 | 0.67 (0.44 – 1.01) | 1.23 (0.87 – 1.75) | 0.69 (0.45 – 1.03) | 1.06 (0.78 – 1.42) |
| Quartile 4 | 1.00 | 1.00 | 1.00 | 1.00 |
| Health insurance, none | 0.39 (0.10 – 1.50) | 1.22 (0.59 – 2.51) | 0.38 (0.10 – 1.45) | 1.25 (0.61 – 2.58) |
| Married (versus other) | 1.10 (0.76 – 1.59) | 0.99 (0.75 – 1.30) | 1.08 (0.75 – 1.57) | 0.99 (0.75 – 1.30) |
| Rheumatoid arthritis | ||||
| Disease duration, per 5-year decrease | 1.11 (1.01 – 1.22) | 0.96 (0.91𢀓 1.02) | 1.11 (1.01 – 1.21) | 0.97 (0.91– 1.02) |
| HAQ, per unit increase | 1.17 (0.89 – 1.53) | 0.98 (0.79 – 1.21) | 1.12 (0.86 – 1.48) | 1.01 (0.82 – 1.25) |
| TJC per increase | 1.03 (0.98 – 1.09) | 1.03 (1.00 – 1.07) | 1.03 (0.98 – 1.08) | 1.03 (0.99 – 1.07) |
| SJC, per increase | 1.06 (0.99 – 1.13) | 1.01 (0.97 – 1.06) | 1.06 (0.99 – 1.13) | 1.01 (0.97 – 1.06) |
| Use of oral steroids | 1.91 (1.36 – 2.67) | 1.18 (0.93 – 1.49) | 1.66 (1.18 – 2.35) | 1.24 (0.93 – 1.57) |
| Comorbidities | ||||
| GDS > 7 | 1.00 (0.64 – 1.56) | 1.19 (0.85 – 1.65) | 1.01 (0.65 – 1.58) | 1.18 (0.84 – 1.65) |
| Comorbidities, none | 1.21 (0.76 – 1.91) | 0.96 (0.91 – 1.02) | 1.22 (0.77 – 1.92) | 0.95 (0.67 – 1.34) |
| Comorbidites, one | 1.07 (0.68 – 1.70) | 0.91 (0.65 – 1.29) | 1.06 (0.67 – 1.68) | 0.93 (0.66 – 1.31) |
| Comorbidities, two+ | 1.00 | 1.00 | 1.00 | 1.00 |
| Hospitalized in past year | 0.87 (0.63 – 1.20) | 1.24 (0.98 – 1.57) | 0.84 (0.61 – 1.16) | 1.24 (0.98 – 1.58) |
| Rheumatology visit | ||||
| None | … | … | 1.00 | 1.00 |
| 1–6 | … | … | 1.70 (1.16 – 2.48) | 0.46 (0.30 – 0.71) |
| > 6 | … | … | 2.15 (1.29 – 3.57) | 0.34 (0.22 – 0.52) |
Notes: Variables in bold are statistically significant.
Includes Pacific Islanders. All models also include year of observation.
Abbreviations: DMARD, disease-modifying anti-rheumatic drug; HMO, health maintenance organization; PPO, preferred provider organization; HAQ, Health Assessment Questionnaire; Rx, prescription medications; RA, rheumatoid arthritis; TJC, tender joint count; SJC, swollen joint count; GDS, geriatric depression scale.
We examined different domains of potential predictors, including RA-related factors, socioeconomic factors and comorbidities (see Supplemental Tables 3 and 4). The c-statistics for RA-related factors were 0.62 for starting a DMARD and 0.60 for stopping a DMARD. Including sociodemographic factors and comorbidities in the fully adjusted models improved the model fit for both sets of models – c-statistics 0.69 for starting a DMARD and 0.68 for stopping a DMARD.
After including rheumatology visits in the prior year as a covariate in the full multivariable models, the predictors and their ORs changed slightly (see Table 2). Several variables were associated with an increased probability of starting DMARDs: greater number of rheumatology visits (OR 2.15, 95% CI 1.29 – 3.57 for > 6 visits compared with 0 visits), younger age (OR 1.28, 95% CI 1.12 – 1.48 per decade), African American race (OR 0.40, 95% CI 0.16 – 0.99), and the use of oral glucocorticoids (OR 1.66, 95% CI 1.18 – 2.35). Predictors of stopping DMARDs in multivariable models included fewer rheumatology visits (OR 2.96, 95% CI 1.87 – 4.60 for zero visits compared with > six visits), older age (OR 1.12, 95% CI 1.01 – 1.24 per decade), Hispanic ethnicity (OR 1.59, 95% CI 1.06 – 2.38), and lowest annual income compared with highest (OR 1.61, 95% CI 1.04 – 2.49).
DISCUSSION
While clinical guidelines strongly support the use of DMARDs for all patients with RA, many studies have noted that their use is not ubiquitous. (2–6) Some studies reporting low rates may include patients who have not been diagnosed with RA by a rheumatologist. We examined DMARD use patterns in a large well-established longitudinal cohort from Northern California, the UCSF RA Panel, who all received an RA diagnosis by a rheumatologist. The focus of this study was to assess patterns and predictors of starting and stopping DMARDs, examining pairs of years to determine if patient factors in the first year predicted the starting or stopping of these agents. Among patients not using DMARDs in a given year, approximately 15% started them in the next year; this rate varied during the follow-up but without a consistent trend. Year-to-year stopping of all DMARDs dropped significantly during the study period, from 9% to 3%. RA-related variables as well as sociodemographic variables were significant predictors of starting and stopping DMARDs.
A surprising aspect of our analysis was that some of the same variables -- Hispanic ethnicity, use of oral glucocorticoids, and more tender joints (trend but not statistically significant) -- predicted both starting and stopping DMARDs. These factors may correlate with frequent treatment switches. It may also be the case that oral glucocorticoid use and more tender joints may motivate some patients not using DMARDs to start them, with oral glucocorticoids used as a “bridge” therapy in such patients. Conversely, patients using DMARDs who also require oral glucocorticoids and report more tender joints may decide to discontinue them if they perceive inadequate therapeutic benefits. The association of Hispanic ethnicity with higher rates of starting and stopping DMARDs may relate to differences in the availability of new therapies (15) coupled with less stable insurance coverage for medications (16) or limited English proficiency that may impair communication with physicians about medication side effects. (17) The higher rate of stopping DMARDs among lower income patients suggests that drug or visit costs may contribute to socioeconomic disparities in DMARD use despite clinical guidelines supporting their use.
The trends in starting and stopping DMARDs over the 23 years of the study are noteworthy. The gradual reduction in subjects stopping DMARDs may correlate with the increasing range of treatment options since the latter half of the 1990s. It may also be that rheumatologists have become less concerned about slightly abnormal laboratory results that occur occasionally by chance, for example liver function tests among methotrexate users. In contrast, we did not observe strong trends in DMARD starting. The apparent swings in the starting DMARD pattern observed around 1998–1999 and 2002–2003 (see Figure 2) may be accounted for by the periodic launches of TNF antagonists during the study period – etanercept and infliximab in late 1998–1999 and adalimumab in 2002. Finally, visits to rheumatologists was the strongest predictor of DMARD starting and stopping, consistent with prior literature. (2, 4)
It is notable that the models’ discrimination (c-statistic) was relatively poor. Even the fully adjusted models, with all types of variables, had c-statistics of only 0.69 (starting) and 0.68 (stopping). We suspect that unmeasured factors, including patients’ personal preferences, real or perceived adverse events, and informational needs, likely contribute to starting and stopping DMARDs.(18) We did find that non-RA related factors were as important as RA related factors, consistent with prior literature suggesting that age, income, race, and ethnicity all correlate with DMARD use. (2–5)
This study has important strengths. The subjects were all diagnosed with RA by rheumatologists. The UCSF RA Panel Study collects a robust set of potential predictors of DMARD use, and they have been collected in a consistent manner annually over many years. Study limitations include the lack of a standardized set of classification criteria to diagnose RA. However, all patients had been diagnosed with RA by a rheumatologist. All subjects were recruited from a geographically concentrated area, thereby limiting the generalizability of the sample. The medication questionnaire used has not been previously validated in patients with RA, and it is possible that the annual self-report of medications might be inaccurate. This should not introduce systematic bias. As well, we did not include the medication history as a potential predictor. Virtually all patients used a DMARD at some point and few patients had incident RA and were new DMARD starters, so this addition of such a variable would not influence the predictive models. It is possible that a given rheumatologist might influence starting or stopping DMARDs. Many patients changed rheumatologists throughout the course of the study period, so this could not be accounted for. In addition, the joint counts were self-reported by subjects, but prior research has validated such methods. (19)
In conclusion, rates of stopping all DMARDs from one year to the next have decreased in frequency among patients with RA since 1982. Predictors of starting and stopping DMARDs include RA-related and sociodemographic variables, such as age, income, and ethnicity. While there are data regarding correlates of DMARD use in the literature, there are no published data that we could identify that describe predictors of starting or stopping DMARDs. Our results inform current efforts to improve the consistency of DMARD use among patients with RA. These findings suggest that efforts to improve DMARD use will likely require improved economic access to rheumatologic care and DMARDs, two potential sources of disparities that may be barriers to consistent DMARD use. As health systems currently evolve with Medicaid expansions and a greater emphasis on primary care, reducing these barriers to appropriate care for rheumatologic patients will be an important goal.
Supplementary Material
INNOVATION.
-
+
Patients with RA frequently start and stop DMARDs.
-
+
DMARD stopping has become less common over the period 1985 – 2005.
-
+
DMARD starting and stopping is related to specific patient characteristics, some disease-related but others not.
-
+
These results will inform current efforts to improve the consistency of DMARD use among patients with RA.
Acknowledgments
Support: National Institutes of Health (NIAMS R01 AR 056215, NIAMS K24 AR 055989, NIAMS P60 AR 047782, NIAMS K23 AR 059677).
Potential Conflicts of Interest: Dr. Solomon receives salary support from research grants to his institution from Amgen, CORRONA, Eli Lilly, and Pfizer. He has served in unpaid roles on trials funded by Pfizer, Eli Lilly, and Novartis. Dr. Brookhart receives salary support from research grants from Amgen. He has served as a scientific advisor to Amgen, Rockwell Medical, Merck, and Pfizer (honoraria declined or paid to institution).
REFERENCES
- 1.Guidelines for the management of rheumatoid arthritis: 2002 Update. Arthritis Rheum. 2002;46(2):328–346. doi: 10.1002/art.10148. [DOI] [PubMed] [Google Scholar]
- 2.Schmajuk G, Schneeweiss S, Katz JN, Weinblatt ME, Setoguchi S, Avorn J, et al. Treatment of older adult patients diagnosed with rheumatoid arthritis: improved but not optimal. Arthritis Rheum. 2007;57(6):928–934. doi: 10.1002/art.22890. [DOI] [PubMed] [Google Scholar]
- 3.Schmajuk G, Trivedi AN, Solomon DH, Yelin E, Trupin L, Chakravarty EF, et al. Receipt of disease-modifying antirheumatic drugs among patients with rheumatoid arthritis in medicare managed care plans. Jama. 2011;305(5):480–486. doi: 10.1001/jama.2011.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Solomon DH, Ayanian JZ, Yelin E, Shaykevich T, Brookhart MA, Katz JN. Use of disease-modifying medications for rheumatoid arthritis by race and ethnicity in the National Ambulatory Medical Care Survey. Arthritis Care Res (Hoboken) 2012;64(2):184–189. doi: 10.1002/acr.20674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grijalva CG, Chung CP, Stein CM, Mitchel EF, Jr, Griffin MR. Changing patterns of medication use in patients with rheumatoid arthritis in a Medicaid population. Rheumatology (Oxford) 2008;47(7):1061–1064. doi: 10.1093/rheumatology/ken193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Khanna R, Smith MJ. Utilization and costs of medical services and prescription medications for rheumatoid arthritis among recipients covered by a state Medicaid program: a retrospective, cross-sectional, descriptive, database analysis. Clin Ther. 2007;29(11):2456–2467. doi: 10.1016/j.clinthera.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 7.Kim SY, Servi A, Polinski JM, Mogun H, Weinblatt ME, Katz JN, et al. Validation of rheumatoid arthritis diagnoses in health care utilization data. Arthritis Res Ther. 2011;13(1):R32. doi: 10.1186/ar3260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lacaille D, Anis AH, Guh DP, Esdaile JM. Gaps in care for rheumatoid arthritis: a population study. Arthritis Rheum. 2005;53(2):241–248. doi: 10.1002/art.21077. [DOI] [PubMed] [Google Scholar]
- 9.Yelin EH, Shearn MA, Epstein WV. Health outcomes for a chronic disease in prepaid group practice and fee for service settings. The case of rheumatoid arthritis. Med Care. 1986;24(3):236–247. doi: 10.1097/00005650-198603000-00006. [DOI] [PubMed] [Google Scholar]
- 10.Yelin EH, Criswell LA, Feigenbaum PG. Health care utilization and outcomes among persons with rheumatoid arthritis in fee-for-service and prepaid group practice settings. Jama. 1996;276(13):1048–1053. [PubMed] [Google Scholar]
- 11.Yelin EH, Henke CJ, Kramer JS, Nevitt MC, Shearn M, Epstein WV. A comparison of the treatment of rheumatoid arthritis in health maintenance organizations and fee-for-service practices. N Engl J Med. 1985;312(15):962–967. doi: 10.1056/NEJM198504113121506. [DOI] [PubMed] [Google Scholar]
- 12.Aday LA, Andersen R. A framework for the study of access to medical care. Health Serv Res. 1974;9(3):208–220. [PMC free article] [PubMed] [Google Scholar]
- 13.Fries JF. The hierarchy of quality-of-life assessment, the Health Assessment Questionnaire (HAQ), and issues mandating development of a toxicity index. Control Clin Trials. 1991;12(4) Suppl:106S–117S. doi: 10.1016/s0197-2456(05)80016-9. [DOI] [PubMed] [Google Scholar]
- 14.Sheikh JI, Yesavage JA, Brooks JO, 3rd, Friedman L, Gratzinger P, Hill RD, et al. Proposed factor structure of the Geriatric Depression Scale. Int Psychogeriatr. 1991;3(1):23–28. doi: 10.1017/s1041610291000480. [DOI] [PubMed] [Google Scholar]
- 15.Wang J, Zuckerman IH, Miller NA, Shaya FT, Noel JM, Mullins CD. Utilizing new prescription drugs: disparities among non-Hispanic whites, non-Hispanic blacks, and Hispanic whites. Health Serv Res. 2007;42(4):1499–1519. doi: 10.1111/j.1475-6773.2006.00682.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shah NS, Carrasquillo O. Twelve-year trends in health insurance coverage among Latinos, by subgroup and immigration status. Health Aff (Millwood) 2006;25(6):1612–1619. doi: 10.1377/hlthaff.25.6.1612. [DOI] [PubMed] [Google Scholar]
- 17.Fernandez YGE, Franks P, Jerant A, Bell RA, Kravitz RL. Depression treatment preferences of Hispanic individuals: exploring the influence of ethnicity, language, and explanatory models. J Am Board Fam Med. 2011;24(1):39–50. doi: 10.3122/jabfm.2011.01.100118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barber N, Parsons J, Clifford S, Darracott R, Horne R. Patients' problems with new medication for chronic conditions. Qual Saf Health Care. 2004;13(3):172–175. doi: 10.1136/qshc.2003.005926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stucki G, Liang MH, Stucki S, Bruhlmann P, Michel BA. A self-administered rheumatoid arthritis disease activity index (RADAI) for epidemiologic research. Psychometric properties and correlation with parameters of disease activity. Arthritis Rheum. 1995;38(6):795–798. doi: 10.1002/art.1780380612. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.