Figure 2. MALDI analysis of human neutrophil Cyt b following modification with mass spectrometry compatible crosslinkers.
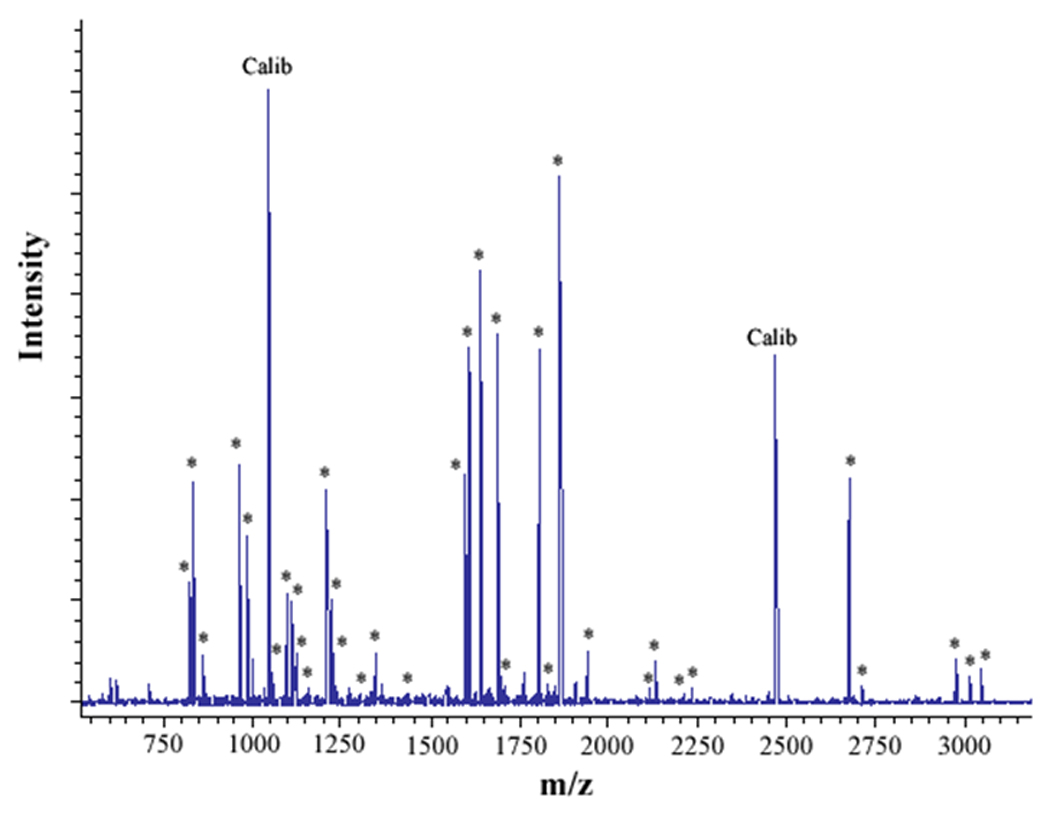
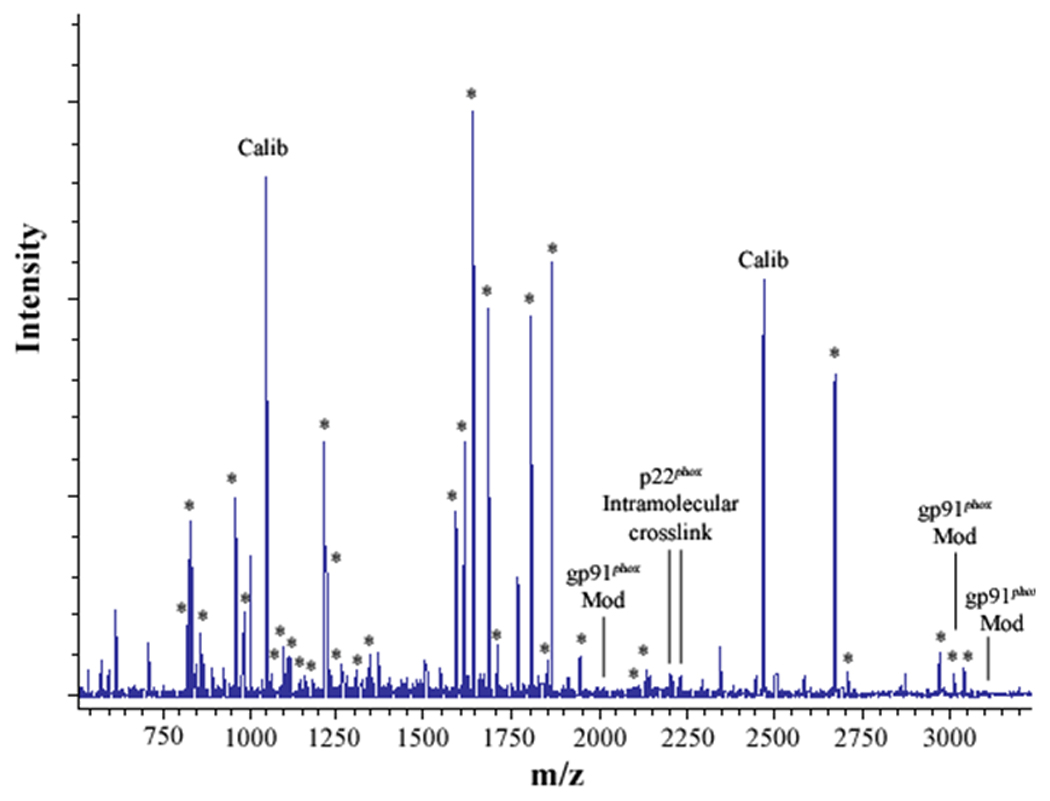
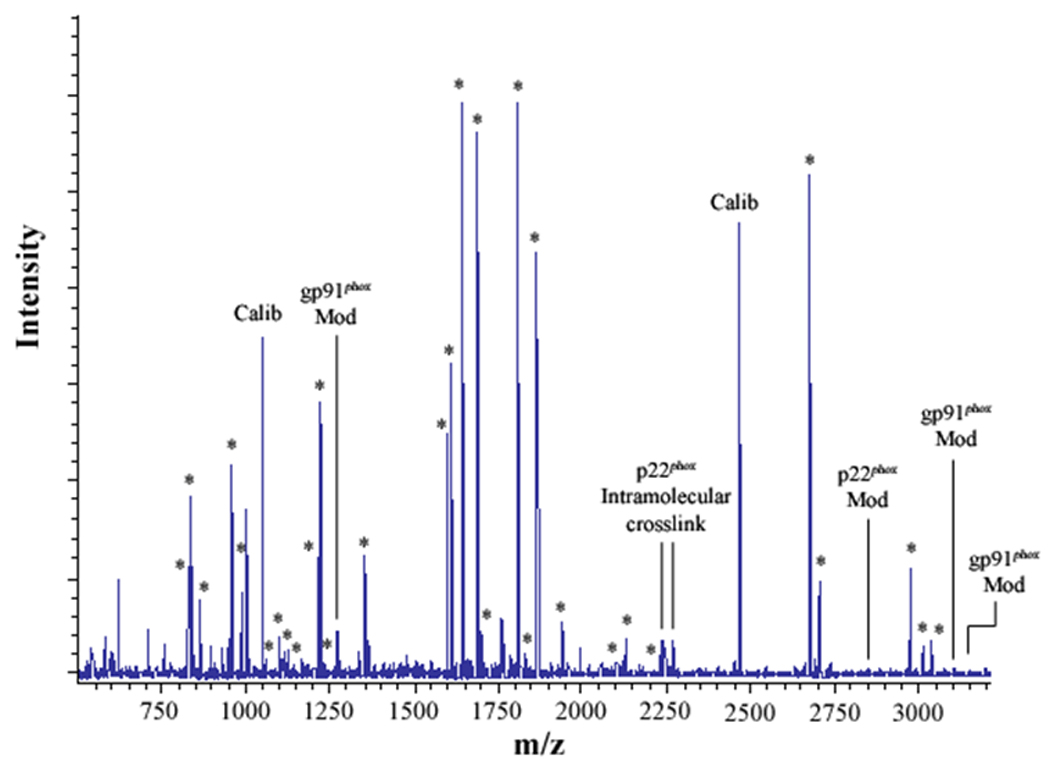
Following chemical modification as outlined in Figure 1, reactions were quenched and digested with Trypsin-gold. To prepare samples for analysis by MALDI mass spectrometry, reactions were reduced, alkylated and then purified using C18ZipTips. (2A) MALDI analysis of Cyt b tryptic digests following incubation with 5% DMSO for 50 min at room temperature (control sample). (2B) MALDI analysis of Cyt b tryptic digests following incubation with a 60-fold molar excess of BS2G-d0/d4 for 50 min at room temperature. (2C) MALDI analysis of Cyt b tryptic digests following incubation with a 60-fold molar excess of BS3-d0/d4 for 50 min at room temperature. Masses attributed to gp91phox and p22phox tryptic peptides are labeled with asterisks (Figure 2A–C), calibration peptides are labeled with text (Calib), while both modified peptides and an intramolecular p22phox crosslink are designated with text in Figures 2B and 2C. Qualitatively similar results were obtained for all reaction conditions used in the present study and indicate that the surface of Cyt b was not extensively modified.
