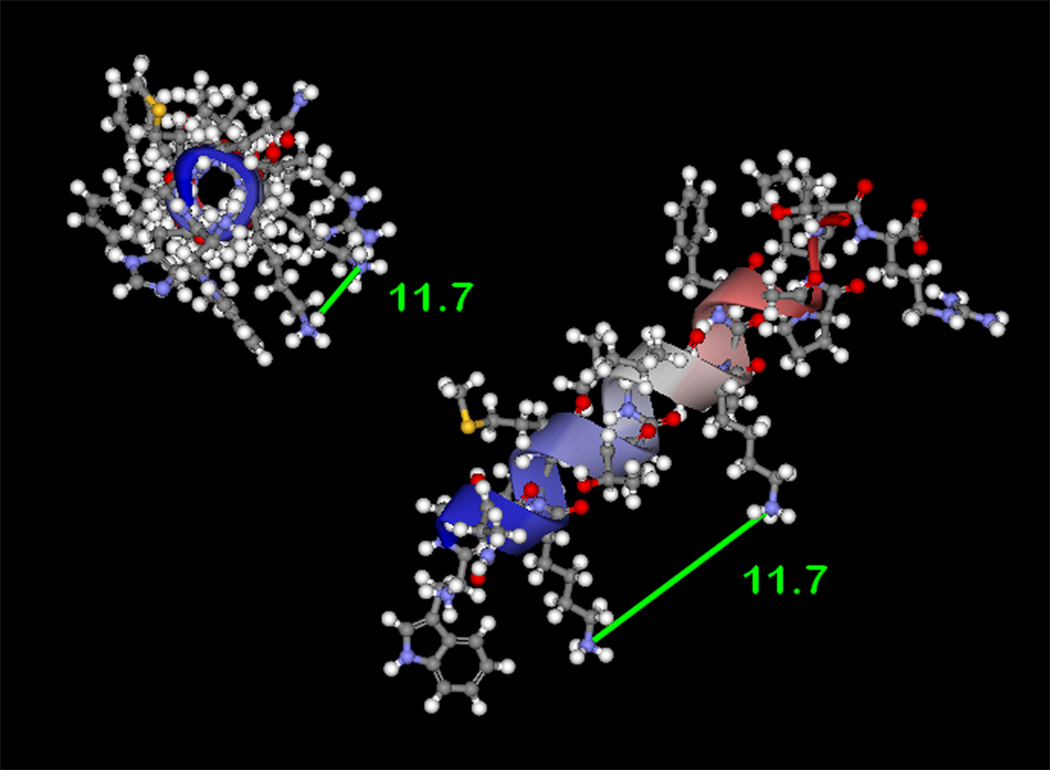
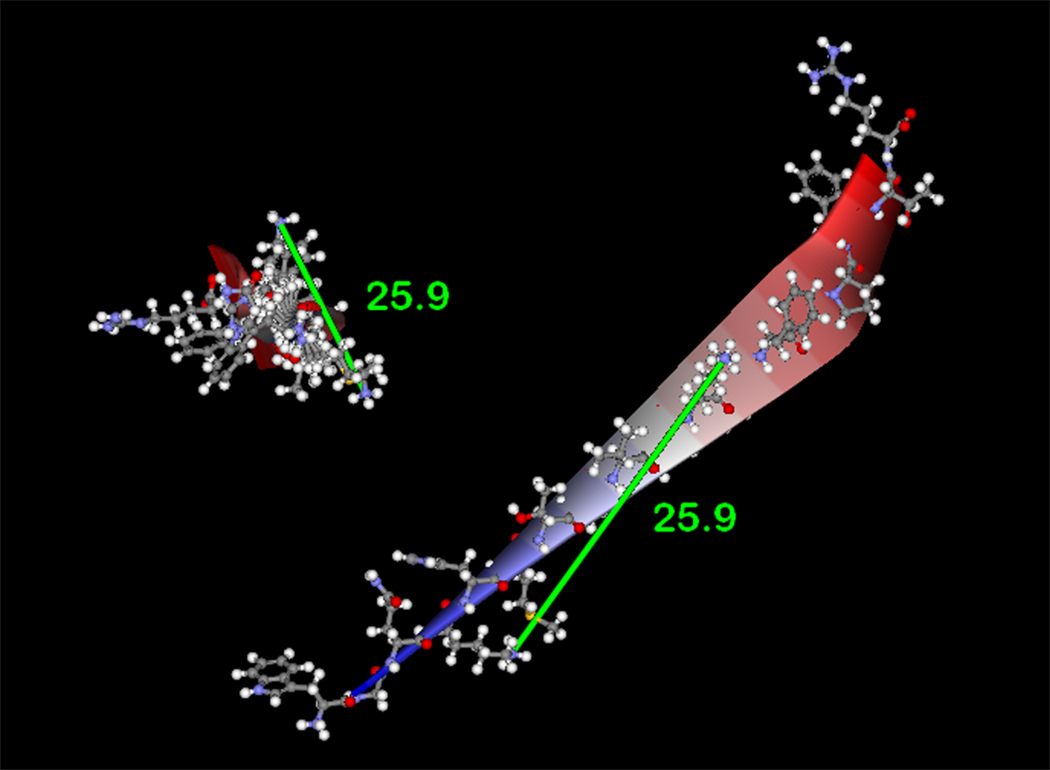
Figure 4. Computational modeling of p22phox residues identified by crosslinking and mass spectrometry.
(4A) Model of p22phox residues 68–85 as an α-helix showing a stacked configuration of Lys71 and Lys78, with a minimized distance between the reactive amines of 11.7 Å. (4B) Model of p22phox residues 68–85 as a β-strand, showing an elongated distance of 25.9 Å between the reactive amine groups of Lys71 and Lys78. In both 4A and 4B, the left structures represent side views of the structure model, while the right structures represent the amino to carboxy-terminal end views. Colorized solid ribbons showing secondary structure are blue and red at the amino and carboxy-terminal ends, respectively, with graded color proceeding in that direction.