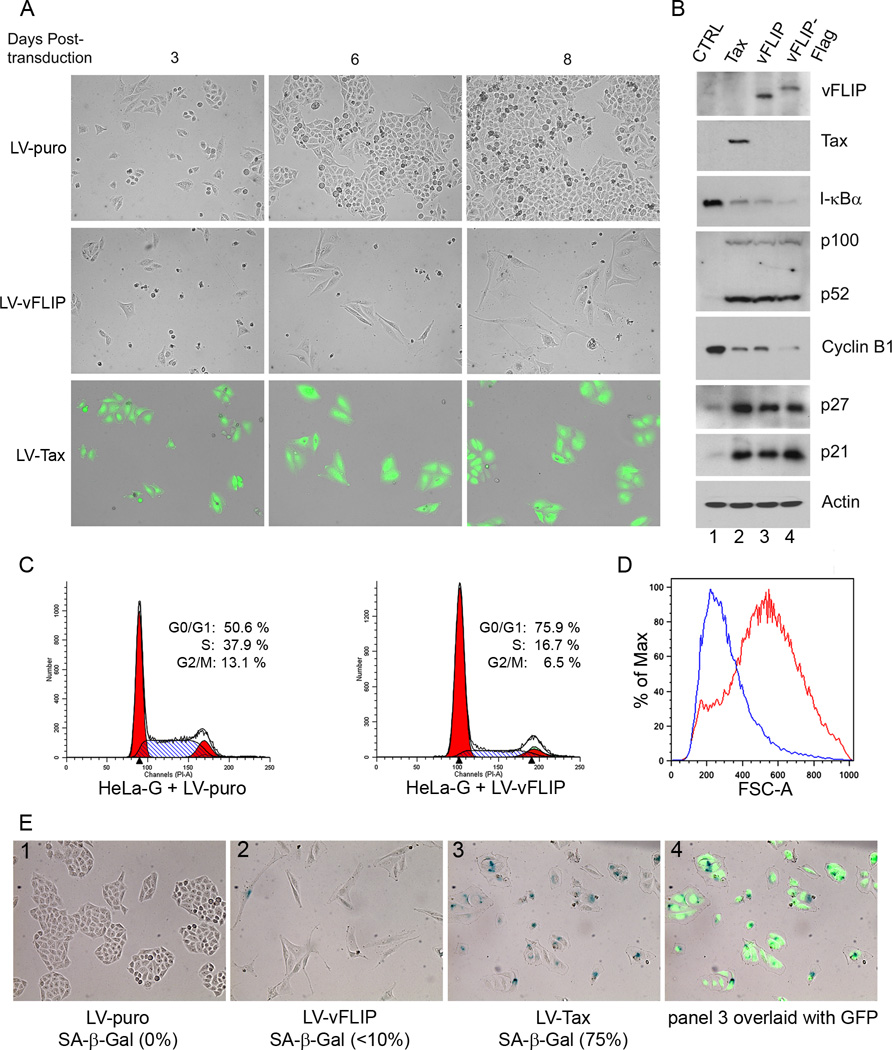
Figure 1. KSHV vFLIP induces G1 cell cycle arrest in HeLa cells.
(A) HeLa-18×21-EGFP (HeLa-G) cells were transduced by LV-puro, LV-vFLIP-puro (LV-vFLIP) or LV-Tax-puro (LV-Tax) lentiviral vector respectively. Forty-eight hours after transduction, cells were seeded sparsely on 6-well plates in fresh medium containing 1 µg/ml puromycin. GFP expression in HeLa-G cells is under the control of 18 copies of a 21-bp Tax-responsive enhancer element that drives abundant GFP expression when Tax is expressed. The transduced cells were monitored microscopically. Photographs were taken at day 3, 6 and 8 after transduction. (B) Cells transduced with the control LV-puro vector (CTRL) and the indicated lentiviral vectors for Tax, vFLIP, and Flag-epitope-tagged vFLIP (vFLIP-Flag) were selected in puromycin for 4 days, and then harvested for immunoblot analysis using antibodies against the proteins indicated on the right of each panel. The relative levels of I-κBα (1; 0.11; 0.08; 0.06), p52 (1; 22; 20; 18), cyclin B1 (1; 0.18; 0.18; 0.08), p27 (1, 8.7; 8.7; 9.3) and p21 (1; 6.6; 5.9; 8.0) in each lanes (lanes 1–4) were determined by densitometry, normalization against β-actin (Actin), and then fold changes computed by normalization again against the corresponding values of lane 1 control (CTRL). (C) Flow cytometry analysis of HeLa-G cells transduced with LV-puro (left panel) or LV-vFLIP-puro (LV-vFLIP, right panel). Transduced cells were selected with 1 µg/ml puromycin for 4 days. Puromycin-resistant cells were then harvested, fixed and stained with propidium iodide for flow cytometry. The percentage of cells in G1, S, and G2/M respectively was determined as in Materials and Methods. (D) The size distribution of cells was determined based on forward scatter and analyzed using the FlowJo software. Blue and red traces represent LV-puro- and LV-vFLIP-transduced cells respectively. (E) Senescence-associated β-gal staining of LV-puro, LV-Tax-puro or LV-vFLIP-puro-transduced cells was carried out as described in Materials and Methods.