Abstract
Inflammation has long been suspected to play a major role in the pathogenesis of cancer. Only recently however, have some mechanisms of its tumor promoting effects come to light. Microbes, both commensal and pathogenic, are critical regulators of the host immune system, and ultimately, of inflammation. Consequently, microbes have the potential power to influence tumor progression as well, through a wide variety of routes, including chronic activation of inflammation, alteration of tumor microenvironment, induction of genotoxic responses, and metabolism. In this review, we will provide a general overview of commensal microbiota, inflammation and cancer, and how microbes fit into this emerging field.
Introduction
The human body quickly becomes inhabited by microorganisms shortly at birth1. Microbes colonize areas that are directly exposed to the air and surroundings, including the mouth, nostrils, skin, stomach, and the gastrointestinal and urogenital tracts2. Each environment favors the survival and growth of particular bacteria, and each bacterial niche thus harbors a characteristic collection of microbes. Nevertheless, there is a large variation in the bacterial composition of sites within each organ system between individuals, and the variability is influenced by genetics, diet, antibiotic and medications intake, and other external environmental factors1-3. Additionally, the immune system affects types and localization of microbiota, through complex regulation of immune tolerance and inflammation.
The composition (quality and quantity) of microbes in the human body is critical to human health. These ecosystems help the body maintain a number of key processes including digestion of complex plant matter, production of high energy metabolites (for example, short chain fatty acids), immune homeostasis, and protection against pathogenic bacterial species2,4,5. Commensal bacteria can outcompete potentially hazardous bacteria by modulating the local environment. The microbiota is thus metabolically active; it exerts its beneficial effects by producing toxins to destroy pathogenic strains of similar species, altering the pH of the local environment6, metabolizing key nutrients to starve their competitors7,8, maintaining mucosal layers and epithelial integrity5,9, and by activating the host immune system10.
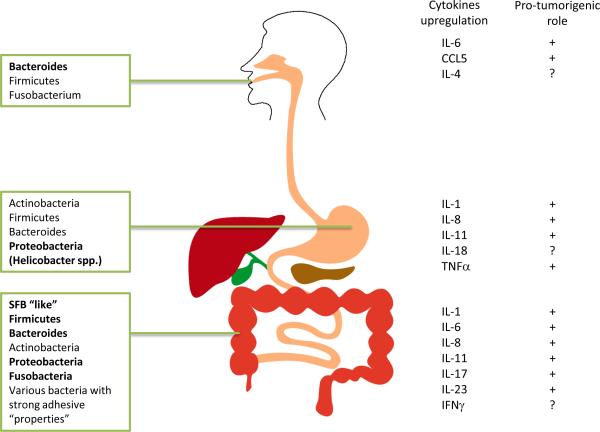
Microbiota diversity is site specific and varies depending on the location in the body, and this diversity (or lack thereof) can correlate with human health. For example, a wide range of commensal microbes in the colon is linked to better health11, while less variety is more beneficial to the overall well being of the vagina12. A pathologic imbalance in a microbial community is referred to as dysbiosis13. Specific pathogens can also take advantage of the altered microbial ratios, or can cause dysbiosis themselves. The ratios of certain phyla of bacteria are known to be significantly shifted in disorders of the skin, colon, and lung2 (Figure 1). For example, in psoriasis, a cutaneous inflammatory condition, there is an increased ratio of Firmicutes vs. Actinobacteria14, and in spontaneous and maternally transmitted colitis mouse models, the numbers of Enterobacteriaceae species are elevated15. Fusobacteria species have been enriched in colon cancer adenomas and tumors16,17. Undoubtedly, dysbiosis is prevalent in diseases of mucosal areas and more research will need to be performed in order to understand the origins of dysbiosis, as well as the mechanisms involved.
Figure 1. Microbiota distribution in the body and its influence on disease.
Normal bacterial composition in various organs of the body (left). Bacterial population increased in dysbiosis are in bold. Cytokines and chemokines upregulated in the process of inflammation and cancer are shown in the right of the figure..
Inflammation and cancer
More than 150 years ago Virchow made the first connection between inflammation and cancer by observing leukocytes in neoplastic tissues18. Recently, evidence of underlying molecular mechanism has been obtained suggesting that inflammation plays an important role in tumorigenesis and that chronic inflammation increases cancer risk19. Up to 10-20% of all cancers can be attributed to infections, often chronic. In more general developmental terms, up to 20% of all cancers are preceded by chronic inflammation at the cancer site as exemplified by hepatocellular carcinoma (HCC) and hepatitis, colon cancer (CAC) and inflammatory bowel disease (IBD), and gastric cancer and H. pylori-induced gastritis 19,20. However, the role of inflammation is not limited to its action during tumor initiation and growth; inflammation can also be induced in growing tumor (“tumor elicited inflammation”) or as a response to anti-cancer therapy and cell death19.
Inflammation preceding cancer development: “textbook” examples
IBD and colon cancer risk
IBD is an important risk factor for colon cancer (CRC) development20, especially in the form called colitis associated cancer (CAC). Indeed, CRC is the third most common malignancy worldwide and is responsible for more than 600,000 deaths per year21. Many cytokines and growth factors up regulated in IBD are also highly expressed in CRC, which are vital for tumor growth. Chronic injury that accompanies IBD induces “wound healing like” reactions important to stimulate pre-neoplastic proliferation. Loss of tissue integrity causes stem cells to be more accessible to mutagens and promotes bacterial-driven inflammation in IBD, CAC, and CRC.
Liver inflammation and risk of cancer
Hepatocellular carcinoma (HCC), the most common form of liver cancer, is the third leading cause of cancer deaths worldwide22. Infections with Hepatitis B (HBV) or C (HBC) viruses increase the risk of HCC by up to 15-17 fold respectively22. Other major risk factors that contribute to HCC are obesity and alcohol consumption23 and 30% of US adults are now estimated to be obese22. While HBV and HCV infections are set to decrease, obesity is clearly on the rise. Obesity results in non-alcoholic fatty liver disease (NAFLD), further progresses to nonalcoholic steatohepatitis (NASH), which leads to cirrhosis (chronic liver disease) and HCC, with obesity overall increasing HCC risk by 5-7 times22. A recent study also showed that translocation of intestinal microbes contributes to hepatic inflammation and fibrosis, in which intestinal microbiota and Toll-like receptors (TLRs) promote HCC24.
Bacteria, stomach inflammation and gastric cancer
Helicobacter pylori is a type of bacterium found in the stomach of about two thirds of the world's population25 and has long been associated with gastric cancer and mucosa-associated lymphoid tissue (MALT) lymphoma26. H. pylori infection is one of the causes of global cancer mortality with 1-3% occurrence in chronically infected individuals26. However, H. pylori is not acting alone to promote gastric cancer. Some studies have shown that H. pylori mono-associated mice developed fewer tumors compared to their germ-free and antibiotic-treated mice27,28. In addition, H. pylori can produce virulence factors such as CagA (cytotoxin-associated gene A), as well as its pathogenic islands (CagPAI) and VacA (vacuolating cytotoxin A), which may dysregulate host intracellular signaling pathways and lower the neoplastic transformation threshold29,. It is known that CagA can interact with host proteins to activate downstream signaling pathways, including MEK/ERK pathway30, NF-κB pathway31, and β-catenin pathway32; thus activating host inflammatory responses and cell proliferation33. Contrasting with to its tumor-promoting effects, H. pylori infection has been associated with lower risks of some other cancers, including esophageal adenocarcinoma in humans2 and gastric cardia cancer25.
Tumor-Elicited Inflammation (TEI)
Even seemingly ‘non-inflammatory’ solid tumors possess a remarkable ability to recruit immune cells and up-regulate pro-inflammatory cytokines and growth factors, which further influence tumor progression and metastasis19,34. This process may be important for further malignant progression and spread of tumors, as well as for regulation of resistance to anti-cancer therapies, even if the initial tumor emergence and growth were not controlled by inflammation. The inflammatory mediator CSF-1, has in particular been demonstrated to be critical in the acceleration of tumor development and in the acquisition of metastatic potential via recruitment of a massive amount of macrophages to pre-malignant areas35. Additionally, tumor expression of oncogenic Ras is thought to be responsible for the up-regulation of the pro-inflammatory cytokine IL-8, which leads to increased tumor size, immune cell infiltration, and angiogenesis in nude mouse models36. Other groups have demonstrated that tumor production of cytokines recruits myeloid cells to the tumor, which in turn secretes IL-6, activating STAT3 and its subsequent downstream pro-oncogenic signaling in tumor cells37,38. We have found that damaged epithelial junctions in colon cancer, due to lack of mucin production and decreased cadherin expression, results in a robust “Th17-like” inflammatory response (IL-23 and its downstream cytokines IL-17, Il-22 and Il-6), exacerbating tumor growth and progression39. Another study highlights that the loss of tumor suppressor p120-catenin, vital to E-cadherin stability and thus to epithelial junctional integrity40 increases expression of GM-CSF, M-CSF, MCP-1, and TNF41, due to disrupted barrier homeostasis. This induces an influx of immature myeloid cells and activated fibroblasts, which continue to support tumor growth.
Not only does inflammation promote primary tumor development, it can also create a metastatic niche in the tumor microenvironment. In mouse model of lung metastasis, lung cancer cells were shown to cause induction of cytokines from bone marrow derived macrophages that promoted metastasis, through a TLR-2 inflammatory mechanism42. Similarly, in an orthotopic breast cancer model, the chemokine CCL2, was found to be a major chemoattractant for inflammatory monocytes, and was critical for the development of a metastatic niche in the lungs, but not for primary tumor development43,44. Blockade of Fas signaling, better known for its role in apoptosis, was recently demonstrated to reduce tumor size and metastatic burden in an orthotopic breast cancer model, by reducing tumor production of IL-6, which inhibited immature myeloid cell accumulation into tumors45. Taken together, inflammatory cytokines and chemokines produced by cancer cells can attract immature myeloid cells or pro-inflammatory T-helper cells into tumors, creating a pro-tumorigenic microenvironment, stimulating cancer cell growth. Simultaneously, the inflammatory signals can foster a metastatic niche in distant organs, paving the road for secondary tumor development. These are just a few examples of the progress made in this resurgent and exciting field known as “TEI”.
Aspirin and NSAIDs-inhibiting TEI
Several studies on the non-steroidal anti-inflammatory drugs (NSAIDs), such as aspirin, on CRC risk have demonstrated that their regular use can reduce CRC incidence by up to 50% 46,47. Additionally, a recent long term study, with 20 years of follow up data, revealed that people who took aspirin (at least 75 mg) regularly had 40-50% reduction in CRC risk, and a 70% reduction of CRC risk was observed if taken for 5 or more years46. Furthermore, in a meta-analysis of four aspirin trials, overall adenoma risk was decreased by 17% over a 3-4 year trial period, while advanced lesions were decreased by 28%. Numerous studies have also determined that non-aspirin NSAIDs reduced CRC risk as well, by as much as 56%, depending on the location of the cancer and the duration of the therapy48, illustrating that inhibition of inflammation is the key.
Presumably, aspirin and other NSAIDs prevent/treat colon cancer by inhibiting the COX-2 (cyclooxygenase-2) enzyme. The major mechanisms by which NSAIDs treat cancer are by either limiting tumor promoting inflammation, or by directly acting on tumor cells, via reduced proliferation and migration. Indeed, selective COX-2 inhibitors, such as Celecoxib, reduced the number of intestinal polyps in patients with FAP (Familial Adenomatous Polyposis) and reduced CRC risk49.
Microbiota and Cancer: Important Mouse Models
While it is well established that inflammation can promote cancer development and progression, and that microbiota is an essential regulator of inflammatory response, a potentially more direct link between microbiota and cancer is incompletely understood. Studies of germ free (gnotobiotic) mice, and various mice with defective immune pathways, have yielded great insight into the role of microbiota and cancer. A study on the IL-10 knockout mouse reveals that the mutant mice develop spontaneous colitis under conventional conditions, but the disease is less severe when mice are housed in specific pathogen free (SPF) conditions50. In a follow up study, the same group discovered that an uncontrolled Th1 response, most likely in response to microbiota, exacerbates colitis in IL-10 deficient mice and results in adenocarcinoma formation in older mutant mice51. Similarly, mice with conditional deletion of STAT3 from macrophages and neutrophils develop chronic colitis, likely through disrupted IL-10 signaling and over active Th1 responses52. These studies suggest that resident commensal bacteria can trigger exaggerated immune responses (colitis) when key components of immune tolerance are broken. Germ free IL-10 KO mice fail to develop colitis, and have no evidence of abnormal immune system activation53, but when colonized with a pathogenic NC101 E. coli strain, they develop tumors much more readily, possibly due to increased DNA damage54.
Additional studies on toll-like receptor (TLR) signaling in mice have also contributed to our understanding of microbes and cancers55. Mice deficient in TLR-4, the major receptor for LPS are much less susceptible to colitis-associated cancer56. Overexpression of TLR-4 in the intestinal epithelium of mice results in hyper-proliferation of crypts and expansion of the stem cell population57. Administration of azoxymethane (AOM) to TLR-4 mice increases β-catenin activation and results in more spontaneous tumors compared to WT mice. Moreover, multiple mouse studies involving the knockout of a key TLR adaptor protein, MyD88, further illustrates the connection between host microbial sensing and the development of cancer58. ApcMin/+ mice devoid of MyD88 have a delayed progression of spontaneous intestinal tumors and reduced expression of inflammatory mediators59. In a skin papilloma and a fibrosarcoma model, MyD88 has also been shown to promote tumorigenesis, presumably through inflammatory cytokines as well60. In all of these models, the over-amplification of inflammation is the promoter of carcinogenesis, and microbiota can also elicit pro-tumorigenic responses. Elevated TLR-4 and MyD88 correlate with poor prognosis in human colon cancer as well61. Thus, microbial sensing through TLR/MyD88 can promote tumor development.
Antibiotic treatment in mouse models has solidified the role of microbiota in cancer, by ameliorating inflammation and limiting cancer progression. This, however, does not imply that prolonged usage of antibiotics may be somehow beneficial for the prevention and treatment of human cancers, apart from those where eradication of single pathogen/carcinogen (i.e. H. pylori) may actually prevent tumor development. Antibiotic depletion of microbiota reduces tumor burden in Nod1−/− mice, which are more prone to developing colitis-associated tumors62. In patients with gastric mucosa-associated lymphoid tissue (MALT) lymphoma, eradication of H. pylori with antibiotics greatly improves the outcome of many patients, and, in some cases, cures them63. In a large analysis of antibiotic trials in patients with IBD, it was demonstrated that broad-spectrum antibiotic treatment ameliorated disease64, confirming that microbiota play an essential role in promoting pro-tumorigenic inflammation. In a colon adenoma mouse model, antibiotic ablation of commensal bacteria reduced tumor burden and inflammatory signature significantly65.
From Microbiota to Cancer Progression: Inflammation as the Link
So how do microbes truly promote cancer development and progression? Do they initiate a protumorigenic microenvironment or are they simply a consequence of cancer? Presumably, the answer lies somewhere in the middle. One commonality across many diseases in which microbiota contribute to progression is the disruption of the mucosal/epithelial layers of organs, allowing bacteria (or bacterial products and their metabolites) to enter compartments that are not normally in close proximity to microbes. This can trigger a local chronic inflammatory response, due to perpetually injured tissue and thus a constant stream of infiltrating microbes/microbial products. For example, in IBD and CRC, the underlying mucosal barrier is disrupted, either by genetic defect or by rapidly expanding tumor cells, exposing the colon tissue and resident immune cells to large amounts of microbial antigens and products65,66. This, in turn, accelerates tumor progression through pro-tumorigenic cytokines and chemokines that can act as growth factors, activate wound-healing programs, induce migration, and promote angiogenesis. A recent study has demonstrated that commensal microbiota induces IL-23 and IL-17, IL-22 and IL-6 signaling in colon adenoma mouse models, due to defects in colon barrier integrity, and antibiotic treatment or genetic ablation of IL-23 abrogates tumorigenesis39. Along these same lines, barrier defects in the intestines of HBUS mice (HB-EGF transgenic mice, predisposed to polyp formation) were shown to allow microbes to induce neutrophil accumulation and inflammation, which promoted cancer development67. Antibiotic treatment could reverse polyp formation, and reintroduction of stool from polyp bearing mice could re-induce polyp formation, indicating the importance of microbiota in neoplastic transformation.
Control of IL-22 signaling has proven to be important in CRC models. IL-18 was shown to down-regulate IL-22BP during injury to the colon, which allowed an increase in IL-22 signaling, that if left unchecked, promoted tumorigenesis68. Similarly, inhibition of IL-22 signaling was shown to reduce inflammation and tumor burden in a microbial driven CRC model69. Antibiotic depletion of commensals results in normalization of colon morphology, increased mucin production, and reduction of infiltrating inflammatory cells, reversing the effects of matriptase depletion70. Commensal E. coli up-regulate IL-17C expression in APCmin/+ mice, as well as in colitis associated cancer mouse model, which increased tumor cell growth through suppression of apoptosis, by induction of BCLXL, and recruitment of tumor promoting lymphocytes71. Ablation of inflammasome proteins, such as NLRP6, selects for “colitogenic” microbes that cause colon inflammation and advanced CAC development, and this is mediated through bacterially induced up-regulation of CCL-5 from epithelial cells, resulting in an influx of IL-6 producing immune cells and increased epithelial proliferation72. Inhibition of IL-6 signaling significantly reduces inflammation and tumor burden, and blocks the effect of transferred colitogenic microbes. These studies support the notion that bacterial localization is critical in the regulation of inflammation in the colon and the breakdown of epithelial or mucosal layer integrity is a major physiological mechanism by which microbiota can promote carcinogenesis.
Clearly, commensals can exacerbate CRC progression, as demonstrated by these studies, antibiotic treatment in cancer mouse models, and inhibition of the microbial sensing pathways (TLR/MyD88)39,58. Recently, several studies have explored the contribution of immune tolerance and commensal microbiota in the colon. T-cell derived IL-10 protects DSS treated APCΔ468 mice from microbe-induced intestinal polyp formation73. Mice with T-cell specific ablation of IL-10 have reduced pro-tumorigenic infiltrating eosinophils, and thus fewer numbers of polyps. Commensals were also shown to help prevent inflammation and, therefore, inflammation-associated tumorigenesis, by promoting a normal wound healing program, characterized by acute inflammation and then epithelial normalization74. Germ free mice have delayed epithelial proliferation, but after roughly one month, exhibit hyper proliferation and no apparent repair of the epithelial layers, while specific pathogen free (SPF) mice are protected from this damage. Interestingly, knockout of TLR/MyD88 in these germ free mice alleviates colitis and stunted tumor growth, suggesting that the TLR/MyD88 pathway may have both microbe dependent and independent mechanisms (danger signals). Therefore, tumor promoting inflammation can be induced in the absence of microbes (in a chemical model) and still promote inflammation associated tumorigenesis, indicating that the requirement for inflammation for tumor growth trumps the potential direct involvement of microbes stimulating tumor growth, i.e. that in many instances, microbes are needed to induce inflammation and do not act directly on the cancerous cells. Therefore, the delicate balance between microbes, host immune system, and inflammation is critical to the development or prevention of cancer.
Pathogenic Bacteria and Cancer
In addition to an imbalance in commensal bacterial composition, pathogenic bacteria play a large role in many diseases, including colorectal cancer. There are a large number of pathogenic microbes known to promote CRC including certain strains of Escherichia coli Streptococcus bovis (now S. gallolyticus), Helicobacter pylori, Bacteroides fragilis, Enterococcus spp, and some members of the Enterobacteriaceae family75,76. These microbes can attach to epithelial layers of the target tissue (colon for example), and directly induce proliferation of epithelial cells, which can lead to hyperplasia. In addition, they can produce toxins that can disrupt the integrity of the epithelial barrier, damage cells, and cause inflammation. As this topic has been highlighted in several recent reviews77-79, we will briefly discuss a few key examples of how pathogenic bacteria can influence cancer progression. E. coli is one of the most extensively studied microbes in the context of CRC. A direct link between E. coli and its attachment and infiltration of tumors has been established, which correlates with poor prognosis in humans80-82. E. coli can promote tumor progression through attachment to colonic epithelial cells, causing hyper-proliferation and inflammation82. In addition, key virulence factors exert pro-tumorigenic effects by damaging DNA or the mucosal layer/epithelial barrier. One major source of toxicity is produced by the polyketide synthase (pks) genotoxicity island. E. coli that are pks+ promote colon cancer development in IL-10−/− murine models and are highly enriched in colon cancer patients54,83. Enterotoxigenic bacteroides fragilis (ETBF) is another bacteria that generates a toxin, leading to CRC promotion and progression84. The B. fragilis toxin can induce a variety of potentially pro-tumorigenic responses, including cleavage of E-cadherin and activation of β-catenin signaling, stimulation of the NF-κB pathway, and induction of Th17 immune responses85,86. Clinically, these findings are relevant as increased Th17 cell infiltration and ETBF colonization into colon tumors correlates with cancer progression and poor outcome of colorectal cancer87. E. coli and ETBF are just two of the many important pathogenic microbes that exemplify the contribution that specific bacterial toxins make to CRC development and progression. In a nice mechanistic study, Fusobacteria were shown to promote colon cancer tumorigenesis by binding to E-cadherin on tumor cells through its FadA adhesin protein, causing stimulation of growth of cancer cells, as well as the ability of the bacteria to invade neighboring tissue, eliciting a pro-tumorigenic immune response88. Further work is needed to determine if the elimination of certain pathogenic isolates in human CRC patients will yield therapeutic results. In the next section, we will discuss possible ways of modulating bacterial composition, virulence factors, and metabolism in an effort to restore microbial balance and alleviate tumor-promoting inflammation.
Therapeutics Targets: Pre/Probiotics, Diet, and Targeting Microbes
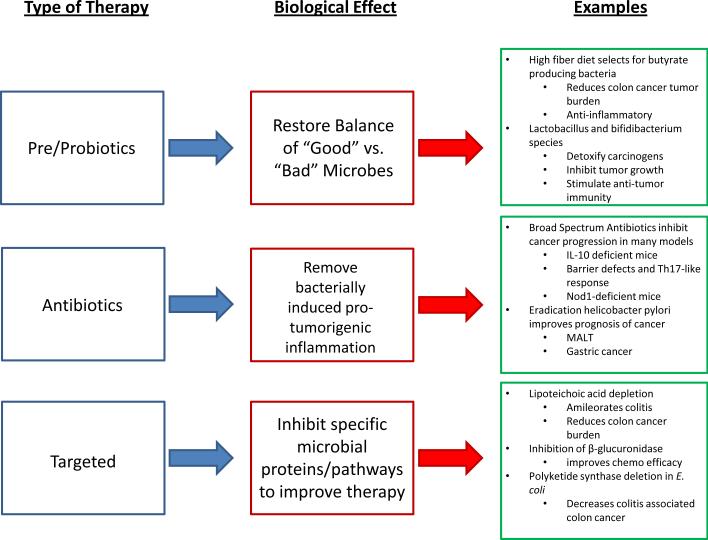
A better understanding of cancer development has afforded investigators the rationale to explore the novel mechanism-based targeted and systemic therapies (Figure 2). Among potential approaches that are not yet fully explored are the usage of probiotics, which aims to “normalize” or “skew’ host microbiome to influence cancer development.
Figure 2. Microbes and Cancer: Therapeutic Avenues.
Three potential intervention points to improve patient responses against cancer, along with what the desired effect, and important examples from the literature. (Author: Need reference(s)?)
Pre/Probiotics
The popularity of prebiotics (a nondigestible food ingredient that selectively stimulates growth of one or a limited number of beneficial colonic bacteria) or probiotics (live microorganisms which confer a health benefit on the host) usage in prevention and treatment of a variety of diseases has increased in the recent years. It is also becoming a progressively crucial part in every day diet, as their beneficial effects are being actively investigated 89,90. One cross-sectional study reports on associations between self-reported dietary fiber intake and the presence of fecal butyrate producing bacteria in subjects with and without advanced colorectal adenomas, raising the possibility that diets low in fiber impact butyrate producing bacteria and short chain fatty acid synthesis and that the resultant alteration in the gut microbiota is related to the presence of colon adenomas90. In addition, consumption of a fiber-rich diet enhances microbial methanogenesis, leading to reduction in hydrogen-producing bacteria, which is remarkable because hydrogen excess in the colon damages NAD regeneration89. Another value of soluble fiber consumption is that it induces a beneficial shift in gut microbiota, particularly Faecalibacterium prausnitzii, a bacterium thought to have anti-inflammatory properties91. The most common probiotic strains used in such treatment are Lactobacillus and Bifidibacterium species. They increase the activity of detoxification of toxin metabolites and carcinogens in colon92, stimulate the host anti-tumor immunity93, produce anti-tumorigenic or anti-mutagenic compounds that interact directly with tumor cells and inhibit their growth94,95 and produce short-chain fatty acids, such as butyrate, which are important for proper immune system regulation.
Targeting Microbes
Although probiotics are becoming an extensively studied field, much less work has been done in developing therapeutics to specifically target microbial pathogenic pathways. Nevertheless, there are some excellent potential targets that could inhibit specific bacterial proteins without upsetting the overall host-microbiota homeostasis. One nicely demonstrated example is in animals infected with lipoteichoic-acid (LTA)-deficient Lactobacillus acidophilus strain, in whom the development of colitis was ameliorated and cancer burden was reduced96. Another group of investigators similarly reported a reduction in colitis-associated colon cancer burden when animals were infected with pks-deleted E. coli strain54. These studies have demonstrated a proof of principle that depletion of a bacterial protein can alleviate disease symptoms. One research group took this a step further and affected host health by targeting a bacterial enzyme. The cancer chemotherapy drug irinotecan can cause severe diarrhea that some patients limits effective therapy. Wallace et al. generated specific inhibitors against the gut bacterial enzyme β-glucuronidase, which reactivates (deconjugates) the conjugated form of irinotecan and causes diarrhea in patients97. The specific inhibition of bacterial β-glucuronidase reduced the toxic side effects associated with chemotherapy in a mouse model, while not harming commensal bacteria. It will be critical in the future to develop specific inhibitors against potentially oncogenic properties of commensal bacteria without disrupting the delicate balance between microbial families.
Conclusions and Unanswered Questions
All in all, the influence of microbes on human health is immense. The microbiome can truly be considered another “organ.” Bacteria, both commensal and pathogenic, contribute to inflammation and cancer development (Figure 3). The external environment (diet, antibiotics, toxins) has an effect on the composition the human microbiome, by altering the bacterial niches that exist within each tissue. This can lead to dysbiosis and select for microbes that disrupt tissue homeostasis through a number of potential mechanisms, including over amplification of the immune response, activation of epithelial proliferation, and breakdown of the integrity of the barrier. Although we are beginning to unravel the complexities of microbial-induced inflammation that promotes cancer, there are questions that remain to be explored. First, can microbes directly induce carcinogenesis without the assistance from tumor promoting inflammation? Much of the evidence suggests that inflammation is required for tumor development. Second, while the recent microbiome projects have uncovered a great deal about the relative ratios of bacteria in distinct organs of healthy individuals vs. cancer patients, there is little mechanistic insight into how these ratios are maintained and ultimately shift in cancer patients. Are there clear patterns that develop? Can some classes of microorganisms functionally substitute for known beneficial microbes? How does dysbiosis occur? How does the host's genetics/environmental exposure factor into the equation? All of these questions require further investigation. Lastly, novel therapeutics must be developed to target these pathogenic and opportunistic microbes. Moreover, we could generate treatments that are designed to prevent microbes from promoting cancer in the first place, by modulating immune system responses, or by maintaining epithelial barrier integrity. While there is much more to be explored, this field has seen some exciting developments in recent years and we should expect significant progress in the near future. Hopefully, we will take care of our microbial communities as well as any other part of our body, as they are just as beneficial to our overall health, and this could reduce the overall cancer burden, saving millions of lives.
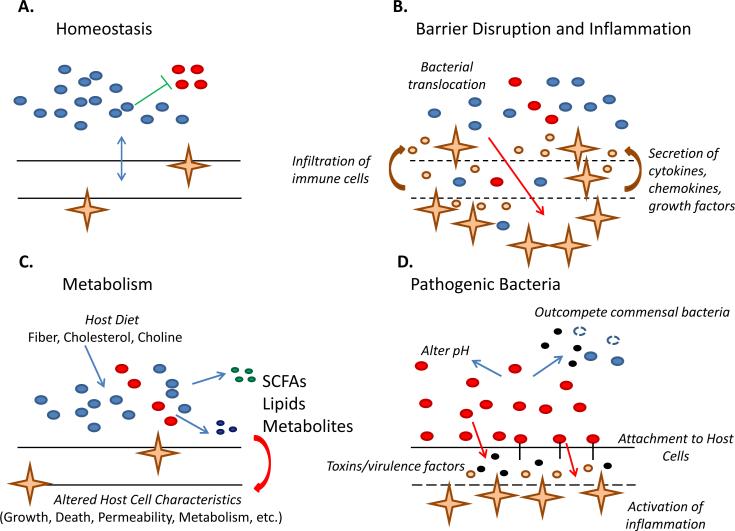
Figure 3. Summary of Microbial Influence on the Host.
A.) Homeostasis. Beneficial bacteria occupy a dominant niche, inhibiting the growth of potentially pathogenic organisms. The integrity of the host organ is maintained and the immune system tolerates and limits bacterial expansion. B.) Barrier Disruption and Inflammation. The barrier integrity of the host organ is compromised, due to tumor growth for example, allowing translocation of bacteria through the barrier, deeper into the organ. This elicits a robust immune response, as immune cells rush into the area, secreting a wide variety of cytokines, chemokines, and growth factors. This can lead to a chronic state of inflammation, and actually support tumor growth. C.) Metabolism. Microbes metabolize dietary intake from the host (fiber, cholesterol, and choline). These are converted into bacterial byproducts, such as short chain fatty acids (SCFAs: butyrate, acetate), lipids, and other metabolites, which modulate host cell behavior. D.) Pathogenic Bacteria. Here, dysbiosis occurs where pathogenic bacteria outcompete commensals by altering the pH and secreting toxins. Some bacteria can attach to the host epithelial layer, or even invade, and induce an immune response. Others actively secrete toxins or possess virulence factors that help break down host tissue and invade further into subsequent layers. The net result is a pro-tumorigenic microenvironment that can promote the progression of cancer.
Acknowledgements
Supported by the NIDDK/NIH R00DK088589, CCFA CDA#2693, FCCC-Temple University Nodal grant and by the Pew Scholar in Biomedical Sciences Program to S.G.
REFERENCES
- 1.Maynard CL, Elson CO, Hatton RD, et al. Reciprocal interactions of the intestinal microbiota and immune system. Nature. 2012;489:231–241. doi: 10.1038/nature11551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cho I, Blaser MJ. The human microbiome: at the interface of health and disease. Nat Rev Genet. 2012;13:260–270. doi: 10.1038/nrg3182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lozupone CA, Stombaugh JI, Gordon JI, et al. Diversity, stability and resilience of the human gut microbiota. Nature. 2012;489:220–230. doi: 10.1038/nature11550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kamada N, Chen GY, Inohara N, et al. Control of pathogens and pathobionts by the gut microbiota. Nat Immunol. 2013;14:685–690. doi: 10.1038/ni.2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Artis D. Epithelial-cell recognition of commensal bacteria and maintenance of immune homeostasis in the gut. Nat Rev Immunol. 2008;8:411–420. doi: 10.1038/nri2316. [DOI] [PubMed] [Google Scholar]
- 6.Ramakrishna BS. Role of the gut microbiota in human nutrition and metabolism. J Gastroenterol Hepatol. 2013;28(Suppl 4):9–17. doi: 10.1111/jgh.12294. [DOI] [PubMed] [Google Scholar]
- 7.Gamage SD, Patton AK, Strasser JE, et al. Commensal bacteria influence Escherichia coli O157:H7 persistence and Shiga toxin production in the mouse intestine. Infect Immun. 2006;74:1977–1983. doi: 10.1128/IAI.74.3.1977-1983.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fabich AJ, Jones SA, Chowdhury FZ, et al. Comparison of carbon nutrition for pathogenic and commensal Escherichia coli strains in the mouse intestine. Infect Immun. 2008;76:1143–1152. doi: 10.1128/IAI.01386-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wlodarska M, Willing B, Keeney KM, et al. Antibiotic treatment alters the colonic mucus layer and predisposes the host to exacerbated Citrobacter rodentium-induced colitis. Infect Immun. 2011;79:1536–1545. doi: 10.1128/IAI.01104-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garrett WS, Gordon JI, Glimcher LH. Homeostasis and inflammation in the intestine. Cell. 2010;140:859–870. doi: 10.1016/j.cell.2010.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Manichanh C, Rigottier-Gois L, Bonnaud E, et al. Reduced diversity of faecal microbiota in Crohn's disease revealed by a metagenomic approach. Gut. 2006;55:205–211. doi: 10.1136/gut.2005.073817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ravel J, Gajer P, Abdo Z, et al. Vaginal microbiome of reproductive-age women. Proc Natl Acad Sci U S A. 2011;108(Suppl 1):4680–4687. doi: 10.1073/pnas.1002611107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Spees AM, Lopez CA, Kingsbury DD, et al. Colonization resistance: battle of the bugs or Menage a Trois with the host? PLoS Pathog. 2013;9:e1003730. doi: 10.1371/journal.ppat.1003730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gao Z, Tseng CH, Strober BE, et al. Substantial alterations of the cutaneous bacterial biota in psoriatic lesions. PLoS One. 2008;3:e2719. doi: 10.1371/journal.pone.0002719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garrett WS, Gallini CA, Yatsunenko T, et al. Enterobacteriaceae act in concert with the gut microbiota to induce spontaneous and maternally transmitted colitis. Cell Host Microbe. 2010;8:292–300. doi: 10.1016/j.chom.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McCoy AN, Araujo-Perez F, Azcarate-Peril A, et al. Fusobacterium is associated with colorectal adenomas. PLoS One. 2013;8:e53653. doi: 10.1371/journal.pone.0053653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Castellarin M, Warren RL, Freeman JD, et al. Fusobacterium nucleatum infection is prevalent in human colorectal carcinoma. Genome Res. 2012;22:299–306. doi: 10.1101/gr.126516.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Virchow R. An Address on the Value of Pathological Experiments. Br Med J. 1881;2:198–203. doi: 10.1136/bmj.2.1075.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883–899. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grivennikov SI. Inflammation and colorectal cancer: colitis-associated neoplasia. Semin Immunopathol. 2013;35:229–244. doi: 10.1007/s00281-012-0352-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tenesa A, Dunlop MG. New insights into the aetiology of colorectal cancer from genome-wide association studies. Nat Rev Genet. 2009;10:353–358. doi: 10.1038/nrg2574. [DOI] [PubMed] [Google Scholar]
- 22.El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology. 2007;132:2557–2576. doi: 10.1053/j.gastro.2007.04.061. [DOI] [PubMed] [Google Scholar]
- 23.Aravalli RN, Steer CJ, Cressman EN. Molecular mechanisms of hepatocellular carcinoma. Hepatology. 2008;48:2047–2063. doi: 10.1002/hep.22580. [DOI] [PubMed] [Google Scholar]
- 24.Dapito DH, Mencin A, Gwak GY, et al. Promotion of hepatocellular carcinoma by the intestinal microbiota and TLR4. Cancer Cell. 2012;21:504–516. doi: 10.1016/j.ccr.2012.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Helicobacter pylori and Cancer. 2013 < http://www.cancer.gov/cancertopics/factsheet/Risk/hpylori-cancer>.
- 26.Fox JG, Wang TC. Inflammation, atrophy, and gastric cancer. J Clin Invest. 2007;117:60–69. doi: 10.1172/JCI30111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lofgren JL, Whary MT, Ge Z, et al. Lack of commensal flora in Helicobacter pylori-infected INS-GAS mice reduces gastritis and delays intraepithelial neoplasia. Gastroenterology. 2011;140:210–220. doi: 10.1053/j.gastro.2010.09.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee CW, Rickman B, Rogers AB, et al. Helicobacter pylori eradication prevents progression of gastric cancer in hypergastrinemic INS-GAS mice. Cancer Res. 2008;68:3540–3548. doi: 10.1158/0008-5472.CAN-07-6786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang F, Meng W, Wang B, et al. Helicobacter pylori-induced gastric inflammation and gastric cancer. Cancer Lett. 2014;345:196–202. doi: 10.1016/j.canlet.2013.08.016. [DOI] [PubMed] [Google Scholar]
- 30.Mueller D, Tegtmeyer N, Brandt S, et al. c-Src and c-Abl kinases control hierarchic phosphorylation and function of the CagA effector protein in Western and East Asian Helicobacter pylori strains. J Clin Invest. 2012;122:1553–1566. doi: 10.1172/JCI61143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brandt S, Kwok T, Hartig R, et al. NF-kappaB activation and potentiation of proinflammatory responses by the Helicobacter pylori CagA protein. Proc Natl Acad Sci U S A. 2005;102:9300–9305. doi: 10.1073/pnas.0409873102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Franco AT, Israel DA, Washington MK, et al. Activation of beta-catenin by carcinogenic Helicobacter pylori. Proc Natl Acad Sci U S A. 2005;102:10646–10651. doi: 10.1073/pnas.0504927102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Suzuki M, Mimuro H, Kiga K, et al. Helicobacter pylori CagA phosphorylation-independent function in epithelial proliferation and inflammation. Cell Host Microbe. 2009;5:23–34. doi: 10.1016/j.chom.2008.11.010. [DOI] [PubMed] [Google Scholar]
- 34.Coussens LM, Zitvogel L, Palucka AK. Neutralizing tumor-promoting chronic inflammation: a magic bullet? Science. 2013;339:286–291. doi: 10.1126/science.1232227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lin EY, Nguyen AV, Russell RG, et al. Colony-stimulating factor 1 promotes progression of mammary tumors to malignancy. J Exp Med. 2001;193:727–740. doi: 10.1084/jem.193.6.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sparmann A, Bar-Sagi D. Ras-induced interleukin-8 expression plays a critical role in tumor growth and angiogenesis. Cancer Cell. 2004;6:447–458. doi: 10.1016/j.ccr.2004.09.028. [DOI] [PubMed] [Google Scholar]
- 37.Fukuda A, Wang SC, Morris J. P. t., et al. Stat3 and MMP7 contribute to pancreatic ductal adenocarcinoma initiation and progression. Cancer Cell. 2011;19:441–455. doi: 10.1016/j.ccr.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lesina M, Kurkowski MU, Ludes K, et al. Stat3/Socs3 activation by IL-6 transsignaling promotes progression of pancreatic intraepithelial neoplasia and development of pancreatic cancer. Cancer Cell. 2011;19:456–469. doi: 10.1016/j.ccr.2011.03.009. [DOI] [PubMed] [Google Scholar]
- 39.Grivennikov SI, Wang K, Mucida D, et al. Adenoma-linked barrier defects and microbial products drive IL-23/IL-17-mediated tumour growth. Nature. 2012;491:254–258. doi: 10.1038/nature11465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Smalley-Freed WG, Efimov A, Burnett PE, et al. p120-catenin is essential for maintenance of barrier function and intestinal homeostasis in mice. J Clin Invest. 2010;120:1824–1835. doi: 10.1172/JCI41414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stairs DB, Bayne LJ, Rhoades B, et al. Deletion of p120-catenin results in a tumor microenvironment with inflammation and cancer that establishes it as a tumor suppressor gene. Cancer Cell. 2011;19:470–483. doi: 10.1016/j.ccr.2011.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kim S, Takahashi H, Lin WW, et al. Carcinoma-produced factors activate myeloid cells through TLR2 to stimulate metastasis. Nature. 2009;457:102–106. doi: 10.1038/nature07623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Qian BZ, Li J, Zhang H, et al. CCL2 recruits inflammatory monocytes to facilitate breast-tumour metastasis. Nature. 2011;475:222–225. doi: 10.1038/nature10138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wolf MJ, Hoos A, Bauer J, et al. Endothelial CCR2 signaling induced by colon carcinoma cells enables extravasation via the JAK2-Stat5 and p38MAPK pathway. Cancer Cell. 2012;22:91–105. doi: 10.1016/j.ccr.2012.05.023. [DOI] [PubMed] [Google Scholar]
- 45.Liu Q, Tan Q, Zheng Y, et al. Blockade of Fas signaling in breast cancer cells suppresses tumor growth and metastasis via disruption of Fas signaling-initiated cancer-related inflammation. J Biol Chem. 2014 doi: 10.1074/jbc.M113.525014. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 46.Rothwell PM, Wilson M, Price JF, et al. Effect of daily aspirin on risk of cancer metastasis: a study of incident cancers during randomised controlled trials. Lancet. 2012;379:1591–1601. doi: 10.1016/S0140-6736(12)60209-8. [DOI] [PubMed] [Google Scholar]
- 47.Thun MJ, Namboodiri MM, Heath CW., Jr. Aspirin use and reduced risk of fatal colon cancer. N Engl J Med. 1991;325:1593–1596. doi: 10.1056/NEJM199112053252301. [DOI] [PubMed] [Google Scholar]
- 48.Ruder EH, Laiyemo AO, Graubard BI, et al. Non-steroidal anti-inflammatory drugs and colorectal cancer risk in a large, prospective cohort. Am J Gastroenterol. 2011;106:1340–1350. doi: 10.1038/ajg.2011.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Steinbach G, Lynch PM, Phillips RK, et al. The effect of celecoxib, a cyclooxygenase-2 inhibitor, in familial adenomatous polyposis. N Engl J Med. 2000;342:1946–1952. doi: 10.1056/NEJM200006293422603. [DOI] [PubMed] [Google Scholar]
- 50.Kuhn R, Lohler J, Rennick D, et al. Interleukin-10-deficient mice develop chronic enterocolitis. Cell. 1993;75:263–274. doi: 10.1016/0092-8674(93)80068-p. [DOI] [PubMed] [Google Scholar]
- 51.Berg DJ, Davidson N, Kuhn R, et al. Enterocolitis and colon cancer in interleukin-10-deficient mice are associated with aberrant cytokine production and CD4(+) TH1-like responses. J Clin Invest. 1996;98:1010–1020. doi: 10.1172/JCI118861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Takeda K, Clausen BE, Kaisho T, et al. Enhanced Th1 activity and development of chronic enterocolitis in mice devoid of Stat3 in macrophages and neutrophils. Immunity. 1999;10:39–49. doi: 10.1016/s1074-7613(00)80005-9. [DOI] [PubMed] [Google Scholar]
- 53.Sellon RK, Tonkonogy S, Schultz M, et al. Resident enteric bacteria are necessary for development of spontaneous colitis and immune system activation in interleukin-10-deficient mice. Infect Immun. 1998;66:5224–5231. doi: 10.1128/iai.66.11.5224-5231.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Arthur JC, Perez-Chanona E, Muhlbauer M, et al. Intestinal inflammation targets cancer-inducing activity of the microbiota. Science. 2012;338:120–123. doi: 10.1126/science.1224820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rakoff-Nahoum S, Medzhitov R. Toll-like receptors and cancer. Nat Rev Cancer. 2009;9:57–63. doi: 10.1038/nrc2541. [DOI] [PubMed] [Google Scholar]
- 56.Fukata M, Chen A, Vamadevan AS, et al. Toll-like receptor-4 promotes the development of colitis-associated colorectal tumors. Gastroenterology. 2007;133:1869–1881. doi: 10.1053/j.gastro.2007.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Santaolalla R, Sussman DA, Ruiz JR, et al. TLR4 activates the beta-catenin pathway to cause intestinal neoplasia. PLoS One. 2013;8:e63298. doi: 10.1371/journal.pone.0063298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Salcedo R, Cataisson C, Hasan U, et al. MyD88 and its divergent toll in carcinogenesis. Trends Immunol. 2013;34:379–389. doi: 10.1016/j.it.2013.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rakoff-Nahoum S, Medzhitov R. Regulation of spontaneous intestinal tumorigenesis through the adaptor protein MyD88. Science. 2007;317:124–127. doi: 10.1126/science.1140488. [DOI] [PubMed] [Google Scholar]
- 60.Swann JB, Vesely MD, Silva A, et al. Demonstration of inflammation-induced cancer and cancer immunoediting during primary tumorigenesis. Proc Natl Acad Sci U S A. 2008;105:652–656. doi: 10.1073/pnas.0708594105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang EL, Qian ZR, Nakasono M, et al. High expression of Toll-like receptor 4/myeloid differentiation factor 88 signals correlates with poor prognosis in colorectal cancer. Br J Cancer. 2010;102:908–915. doi: 10.1038/sj.bjc.6605558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chen GY, Shaw MH, Redondo G, et al. The innate immune receptor Nod1 protects the intestine from inflammation-induced tumorigenesis. Cancer Res. 2008;68:10060–10067. doi: 10.1158/0008-5472.CAN-08-2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Weber DM, Dimopoulos MA, Anandu DP, et al. Regression of gastric lymphoma of mucosa-associated lymphoid tissue with antibiotic therapy for Helicobacter pylori. Gastroenterology. 1994;107:1835–1838. doi: 10.1016/0016-5085(94)90828-1. [DOI] [PubMed] [Google Scholar]
- 64.Wang SL, Wang ZR, Yang CQ. Meta-analysis of broad-spectrum antibiotic therapy in patients with active inflammatory bowel disease. Exp Ther Med. 2012;4:1051–1056. doi: 10.3892/etm.2012.718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Salim SY, Soderholm JD. Importance of disrupted intestinal barrier in inflammatory bowel diseases. Inflamm Bowel Dis. 2011;17:362–381. doi: 10.1002/ibd.21403. [DOI] [PubMed] [Google Scholar]
- 66.Soler AP, Miller RD, Laughlin KV, et al. Increased tight junctional permeability is associated with the development of colon cancer. Carcinogenesis. 1999;20:1425–1431. doi: 10.1093/carcin/20.8.1425. [DOI] [PubMed] [Google Scholar]
- 67.Bongers G, Pacer ME, Geraldino TH, et al. Interplay of host microbiota, genetic perturbations, and inflammation promotes local development of intestinal neoplasms in mice. J Exp Med. 2014;211:457–472. doi: 10.1084/jem.20131587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Huber S, Gagliani N, Zenewicz LA, et al. IL-22BP is regulated by the inflammasome and modulates tumorigenesis in the intestine. Nature. 2012;491:259–263. doi: 10.1038/nature11535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kirchberger S, Royston DJ, Boulard O, et al. Innate lymphoid cells sustain colon cancer through production of interleukin-22 in a mouse model. J Exp Med. 2013;210:917–931. doi: 10.1084/jem.20122308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kosa P, Szabo R, Molinolo AA, et al. Suppression of Tumorigenicity-14, encoding matriptase, is a critical suppressor of colitis and colitis-associated colon carcinogenesis. Oncogene. 2012;31:3679–3695. doi: 10.1038/onc.2011.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Song X, Gao H, Lin Y, et al. Alterations in the microbiota drive interleukin-17C production from intestinal epithelial cells to promote tumorigenesis. Immunity. 2014;40:140–152. doi: 10.1016/j.immuni.2013.11.018. [DOI] [PubMed] [Google Scholar]
- 72.Hu B, Elinav E, Huber S, et al. Microbiota-induced activation of epithelial IL-6 signaling links inflammasome-driven inflammation with transmissible cancer. Proc Natl Acad Sci U S A. 2013;110:9862–9867. doi: 10.1073/pnas.1307575110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dennis KL, Wang Y, Blatner NR, et al. Adenomatous polyps are driven by microbe-instigated focal inflammation and are controlled by IL-10-producing T cells. Cancer Res. 2013;73:5905–5913. doi: 10.1158/0008-5472.CAN-13-1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhan Y, Chen PJ, Sadler WD, et al. Gut microbiota protects against gastrointestinal tumorigenesis caused by epithelial injury. Cancer Res. 2013;73:7199–7210. doi: 10.1158/0008-5472.CAN-13-0827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Collins D, Hogan AM, Winter DC. Microbial and viral pathogens in colorectal cancer. Lancet Oncol. 2011;12:504–512. doi: 10.1016/S1470-2045(10)70186-8. [DOI] [PubMed] [Google Scholar]
- 76.Tjalsma H, Boleij A, Marchesi JR, et al. A bacterial driver-passenger model for colorectal cancer: beyond the usual suspects. Nat Rev Microbiol. 2012;10:575–582. doi: 10.1038/nrmicro2819. [DOI] [PubMed] [Google Scholar]
- 77.Goldszmid RS, Dzutsev A, Trinchieri G. Host Immune Response to Infection and Cancer: Unexpected Commonalities. Cell Host Microbe. 2014;15:295–305. doi: 10.1016/j.chom.2014.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sears CL, Garrett WS. Microbes, Microbiota, and Colon Cancer. Cell Host Microbe. 2014;15:317–328. doi: 10.1016/j.chom.2014.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Schwabe RF, Jobin C. The microbiome and cancer. Nat Rev Cancer. 2013;13:800–812. doi: 10.1038/nrc3610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bonnet M, Buc E, Sauvanet P, et al. Colonization of the Human Gut by E. coli and Colorectal Cancer Risk. Clin Cancer Res. 2014;20:859–867. doi: 10.1158/1078-0432.CCR-13-1343. [DOI] [PubMed] [Google Scholar]
- 81.Martin HM, Campbell BJ, Hart CA, et al. Enhanced Escherichia coli adherence and invasion in Crohn's disease and colon cancer. Gastroenterology. 2004;127:80–93. doi: 10.1053/j.gastro.2004.03.054. [DOI] [PubMed] [Google Scholar]
- 82.Swidsinski A, Khilkin M, Kerjaschki D, et al. Association between intraepithelial Escherichia coli and colorectal cancer. Gastroenterology. 1998;115:281–286. doi: 10.1016/s0016-5085(98)70194-5. [DOI] [PubMed] [Google Scholar]
- 83.Buc E, Dubois D, Sauvanet P, et al. High prevalence of mucosa-associated E. coli producing cyclomodulin and genotoxin in colon cancer. PLoS One. 2013;8:e56964. doi: 10.1371/journal.pone.0056964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sears CL. Enterotoxigenic Bacteroides fragilis: a rogue among symbiotes. Clin Microbiol Rev. 2009;22:349–369. doi: 10.1128/CMR.00053-08. Table of Contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wu S, Morin PJ, Maouyo D, et al. Bacteroides fragilis enterotoxin induces c-Myc expression and cellular proliferation. Gastroenterology. 2003;124:392–400. doi: 10.1053/gast.2003.50047. [DOI] [PubMed] [Google Scholar]
- 86.Wu S, Rhee KJ, Albesiano E, et al. A human colonic commensal promotes colon tumorigenesis via activation of T helper type 17 T cell responses. Nat Med. 2009;15:1016–1022. doi: 10.1038/nm.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tosolini M, Kirilovsky A, Mlecnik B, et al. Clinical impact of different classes of infiltrating T cytotoxic and helper cells (Th1, th2, treg, th17) in patients with colorectal cancer. Cancer Res. 2011;71:1263–1271. doi: 10.1158/0008-5472.CAN-10-2907. [DOI] [PubMed] [Google Scholar]
- 88.Rubinstein MR, Wang X, Liu W, et al. Fusobacterium nucleatum promotes colorectal carcinogenesis by modulating E-cadherin/beta-catenin signaling via its FadA adhesin. Cell Host Microbe. 2013;14:195–206. doi: 10.1016/j.chom.2013.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Turner ND, Ritchie LE, Bresalier RS, et al. The microbiome and colorectal neoplasia: environmental modifiers of dysbiosis. Curr Gastroenterol Rep. 2013;15:346. doi: 10.1007/s11894-013-0346-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chen HM, Yu YN, Wang JL, et al. Decreased dietary fiber intake and structural alteration of gut microbiota in patients with advanced colorectal adenoma. Am J Clin Nutr. 2013;97:1044–1052. doi: 10.3945/ajcn.112.046607. [DOI] [PubMed] [Google Scholar]
- 91.Hooda S, Boler BM, Serao MC, et al. 454 pyrosequencing reveals a shift in fecal microbiota of healthy adult men consuming polydextrose or soluble corn fiber. J Nutr. 2012;142:1259–1265. doi: 10.3945/jn.112.158766. [DOI] [PubMed] [Google Scholar]
- 92.Challa A, Rao DR, Chawan CB, et al. Bifidobacterium longum and lactulose suppress azoxymethane-induced colonic aberrant crypt foci in rats. Carcinogenesis. 1997;18:517–521. doi: 10.1093/carcin/18.3.517. [DOI] [PubMed] [Google Scholar]
- 93.Femia AP, Luceri C, Dolara P, et al. Antitumorigenic activity of the prebiotic inulin enriched with oligofructose in combination with the probiotics Lactobacillus rhamnosus and Bifidobacterium lactis on azoxymethane-induced colon carcinogenesis in rats. Carcinogenesis. 2002;23:1953–1960. doi: 10.1093/carcin/23.11.1953. [DOI] [PubMed] [Google Scholar]
- 94.Han W, Mercenier A, Ait-Belgnaoui A, et al. Improvement of an experimental colitis in rats by lactic acid bacteria producing superoxide dismutase. Inflamm Bowel Dis. 2006;12:1044–1052. doi: 10.1097/01.mib.0000235101.09231.9e. [DOI] [PubMed] [Google Scholar]
- 95.Carroll IM, Andrus JM, Bruno-Barcena JM, et al. Anti-inflammatory properties of Lactobacillus gasseri expressing manganese superoxide dismutase using the interleukin 10-deficient mouse model of colitis. Am J Physiol Gastrointest Liver Physiol. 2007;293:G729–738. doi: 10.1152/ajpgi.00132.2007. [DOI] [PubMed] [Google Scholar]
- 96.Lightfoot YL, Mohamadzadeh M. Tailoring gut immune responses with lipoteichoic acid-deficient Lactobacillus acidophilus. Front Immunol. 2013;4:25. doi: 10.3389/fimmu.2013.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wallace BD, Wang H, Lane KT, et al. Alleviating cancer drug toxicity by inhibiting a bacterial enzyme. Science. 2010;330:831–835. doi: 10.1126/science.1191175. [DOI] [PMC free article] [PubMed] [Google Scholar]