Abstract
Both observations and models suggest that large-scale coastal blooms of Alexandrium fundyense in the Gulf of Maine are seeded by deep-bottom cyst accumulation zones (“seed beds”) where cysts germinate from the sediment surface or the overlying near-bottom nepheloid layers at water depths exceeding 100 m. The germling cells and their vegetative progeny are assumed to be subject to mortality while in complete darkness, as they swim to illuminated surface waters. To test the validity of this assumption we conducted laboratory investigations of cyst viability and the survival of the germling cells and their vegetative progeny during prolonged exposure to darkness at a temperature of 6 °C, simulating the conditions in deep Gulf of Maine waters. We isolated cysts from bottom sediments collected in the Gulf of Maine under low red light and incubated them in 96-well tissue culture-plates in culture medium under a 10:14 h light:dark cycle and under complete darkness. Cyst viability was high, with excystment frequency reaching 90% in the illuminated treatment after 30 days and in the dark treatment after 50 days. Average germination rates were 0.062 and 0.038 d−1 for light and dark treatments, respectively. The dark treatment showed an approximately 2-week time lag in maximum germination rates compared to the light treatment. Survival of germlings was considerably lower in the dark treatment. In the light treatments, 47% of germinated cysts produced germlings that were able to survive for 7 days and produce vegetative progeny, i.e., there were live cells in the well along with an empty cyst at least once during the experiment. In the dark treatments 12% of the cysts produced germlings that were able to survive for the same length of time. When dark treatments are scaled to take into account non-darkness related mortality, approximately 28% of the cysts produced germlings that were able to survive for at least 7 days. Even though cysts are able to germinate in darkness, the lack of illumination considerably reduces survival rate of germling cells. In addition to viability of cysts in surface sediments and the near-bottom nepheloid layer, survivability of germling cells and their vegetative progeny at aphotic depths is an important consideration in assessing the quantitative role of deep-coastal cyst seed beds in bloom formation.
Keywords: Dinoflagellate, Alexandrium fundyense, Cysts, Excystment, Planomeiocyte, Germling, Dark survival, Gulf of Maine
1. Introduction
The life cycle of many dinoflagellates includes a non-motile resting stage (cyst) that remains in bottom sediments or near-bottom nepheloid layers when conditions in the water column are unfavorable for growth (Dale, 1983; Matsuoka and Fukuyo, 2003; Wall, 1971; Kirn et al., 2005; Pilskaln et al., 2014). The switch from cyst to motile cell and vice versa determines the presence of those dinoflagellates in the water column. Numerous studies have shown that germination of dinoflagellate cysts is determined by internal and external factors (Anderson et al., 2005; Rengefors and Anderson, 1998). Among the internal factors, a mandatory dormancy period (maturation) after encystment lasting days to months (Anderson, 1980; Bravo and Anderson, 1994) and, for some species such as Alexandrium fundyense, an annual internal biological clock (Anderson and Keafer, 1987; Matrai et al., 2005a; Perez et al., 1998) regulates germination. Although oxygen is required for germination (Anderson et al., 1987; Kremp and Anderson, 2000; Rengefors and Anderson, 1998), temperature is often viewed as the main environmental determinant for cysts in the surface layer of sediments (Anderson, 1998; Dale, 1983). Light is not necessary for germination in all species but, in some, darkness slows down the process and might reduce the germination frequency and rate (Anderson et al., 2005, 1987; Bravo and Anderson, 1994; Genovesi et al., 2009).
Regarding A. fundyense bloom dynamics in the Gulf of Maine, germination is an important factor determining the initial bloom populations, since few overwintering motile cells have been encountered in surface waters in that region (Anderson et al., 2005; Kirn et al., 2005). Although the germination of A. fundyense has been thoroughly studied (Anderson et al., 2005) there is a lack of information on the fate of the newly germinated cells (germlings) and their immediate vegetative progeny. The germination frequency and viability of germlings and their progeny has been estimated for shallow water populations of A. tamarense in French coastal waters (Genovesi et al., 2009). The authors found high germination frequencies for natural cysts isolated from field samples (85%) but only 27% of the germlings were observed alive 1 day after germination. Of the surviving germlings, 76% were able to divide at least once during a 30-day experiment. The length of cold and dark storage of cysts was observed to influence these ratios, with increasing storage time decreasing the cyst “quality” and thus the ability of the germling and its progeny to survive (Genovesi et al., 2009). The largest cyst seed banks of A. fundyense in the northeastern US lie in the Gulf of Maine at depths >100 m (Anderson et al., 2005; Anderson et al., 2014). In this environment, the viability of cysts, germlings, and their progeny is thus affected by darkness and low temperatures. Darkness has been reported to impair germling survival for other dinoflagellate species (Anderson et al., 1987; Bravo and Anderson, 1994) and it could therefore have an impact on current model parameterizations for A. fundyense in the Gulf of Maine.
Here, we describe a germination and survival experiment with a light and dark treatment in a constant cold temperature (6 °C) performed with A. fundyense cysts collected from the Gulf of Maine. The results show the effect of darkness on cyst germination rates as well as the fate of the newly-germinated cells. Our findings give new insights into factors affecting the bloom-seeding capacity of deep cyst seedbeds. The results can improve estimates of the flux of newly-germinated cells from bottom sediments to the surface layer and thus the output of physical– biological models of A. fundyense population dynamics in the Gulf of Maine (Anderson et al., 2005; McGillicuddy et al., 2005; Stock et al., 2005; He et al., 2008; Li et al., 2009).
2. Materials and methods
2.1. Sample collection and storage
Eight sediment cores were collected using a hydraulically-damped piston corer (Craib, 1965) at a 120 m-deep site in the northwestern Gulf of Maine (43° 36′ N, 69° 22′ W) in October, 2009. The site is situated in a high-density cyst accumulation area (seedbed) along the mid-Maine coast. The cores were sectioned, and slices were collected at 0–1 and 1–3 cm depths in the cores. The eight 0–1 and 1–3 cm slices for each core were pooled to yield one homogenized 0–1 cm and one 1–3 cm sample that were stored in completely-filled 50-mL plastic tubes. Based on sediment color, which was black, the sediment was deemed anoxic. The tubes were stored in the cold (4 °C) and in total darkness immediately after filling. The tubes were stored in these conditions until cyst isolation was conducted on 29 March, 2010 (6 months after collection), allowing the cysts to complete their mandatory maturation period (Anderson, 1980; Bravo and Anderson, 1994) and enter the germination window regulated by an internal clock (Anderson and Keafer, 1987; Matrai et al., 2005a).
2.2. Sample processing, cyst isolation and experimental setup
All sediment processing was done in a darkened laboratory with the only light source being a soft red light. On 29 March, 2010, an aliquot of the cold- and dark-stored sediment from the 1–3 cm layer was taken. To avoid including sediment that might have been oxidized during storage the top layer of sediment from the 50-mL tube was removed and then a sample of arbitrary volume was scooped from the center of the tube, avoiding sediment along the tube walls, into a 50 mL sample tube. The average cyst concentration in the 1–3 cm layer was 2763 cysts cm−3 of wet sediment. Filtered sea water was added to a final volume of 45 mL and the slurry was sonicated with a Branson Sonifier 250 at a constant 40W output for 1 min, and sieved to yield a clean 20–75 µm size fraction (Anderson et al., 2003). The cysts were further concentrated and the sample cleaned of debris with a non-toxic isosmotic density centrifugation method (Schwinghamer et al., 1991). Sucrose and colloidal silica (Nalco) was used to create a solution with two density layers of 1.07 g cm−3 and 1.35 g cm−3. The mode A. fundyense cyst density is 1.2 g cm−3 (Anderson et al., 1985), thus most of the cysts would concentrate at the interface of the two liquids after centrifugation. Prior to centrifugation, 5 mL of the processed sediment sample was added to a 50-mL centrifuge tube. Twenty milliliters of the lighter density gradient solution was then carefully delivered under the processed sediment with a Pasteur pipette, after which 20 mL of the denser solution was delivered under the lighter solution. The tube was centrifuged at 2500 rpm for 20 min at 4 °C and the clear lighter solution was aspirated and the liquid at the interface of the two solutions was collected with a pipette and sieved through a 20 µm sieve. The contents were collected into a 15-mL sample tube which was immediately partitioned out into 1-mL Sedgwick–Rafter slides, and cysts were isolated using micropipettes.
Upon isolation, individual cysts were immediately put into 96- well culture-plates with 300 µL of modified (silica excluded) f/2 medium (Anderson et al., 1994) in each well. It took approximately 45 min to prepare one plate of isolated cysts. After each plate contained approximately 30 isolated cysts, plates were sealed with electrical tape and put in a zip-lock bag along with a moistened paper towel to reduce evaporative loss from the wells. Plates were then incubated in a 6 °C walk-in incubator. In total, we isolated 10 plates (~300 cysts) of which five were designated as dark-treatment plates prior to isolation. The dark-treatment plates were treated similarly to the light treatments, with a few exceptions. Isolations were done in a dark room under low red ambient light and a red filter (Kodak Wratten Red 25) that blocked light <580 nm was placed in front of the light source of the microscope (isolations for the light treatment were done under normal laboratory illumination and no light filtering on the microscope). All other light-emitting areas on the microscope were wrapped in red plastic film or covered. The sealed plates were wrapped in foil and put in a dark colored plastic container that was put inside a black plastic bag before incubation at 6 °C. Light treatment plates were incubated at 6 °C with a 14 h:10 h light:dark cycle with an irradiation of approximately 250 µmol photons m−2 s−1. Cysts incubated in the dark were only exposed to low levels of red light during sediment processing for approximately 30 min, cyst isolation (approximately 45 min at most), and microscopic examination of the plates. For specific wells there was a brief direct exposure during the examination of that well, and then scattered red light during the examination of the rest of the wells. It took approximately 1 h to examine one dark treatment plate during the germination and viability assessments.
2.3. Microscopic examination of isolated cysts
The well plates were checked immediately after they were sealed, using an inverted Zeiss IM35 microscope equipped with a Zeiss 09 filter set (excitation 450–490 nm, emission 515–750 nm) to detect chlorophyll-a (Chl-a) autofluorescence. Dark plates were always shielded from multi-spectral light and examined only under low red light in a darkroom. During this initial examination of the plates, the location and number of cysts in each well were recorded. Wells with multiple cysts were eliminated from the experiment. Cysts in the light treatment were checked for onset of Chl-a autofluorescence, which is considered an indication of pending germination (Anderson and Keafer, 1985; Anderson et al., 2005) and for germination by detecting empty cysts or swimming A. fundyense cells. After cyst germination, we continued to check the plates for swimming cells in order to see how long the germling or the vegetative progeny produced by the germling would survive under our experimental conditions. After cyst germination, the wells were checked by focusing from the bottom to the top of the well several times to observe the swimming cells.
During the first week of the experiment, one light treatment plate was checked on days 1, 2, 3, 4, and 8 to detect the onset of cyst Chl-a autofluorescence. When cysts started displaying auto-fluorescence, the rest of the plates were also checked: the light treatment plates for cyst Chl-a autofluorescence and germination and the dark treatment plates for germination only, since it was not possible to detect Chl-a autofluorescence with red light excitation. During the experiment, light treatment plates were checked on days 1, 2, 3, 4, 8, 9, 11, 12, 15, 18, 22, 29, 36, 44, 50, 57, and 65, and dark treatment plates on days 1, 10, 15, 22, 30, 37, 44, 50, 57, and 65.
2.4. Estimating the germination rate and survival ratio
To quantify germination rates we fitted a simple first-order equation to germination time course data (Anderson et al., 1987). For an initial number of cysts N0: Nt = N0 −kt, where Nt is the number of cysts remaining at time t and k is the specific germination rate with units of time−1. Germination rates are expressed as averages for the duration of the experiment.
The survival ratio of germlings and their vegetative progeny was estimated by recording the occurrence of live cells in tissue culture plate wells once every week. For each weekly observation after cyst germination the number of wells with live cells was divided by the total number of germinated cysts in each plate to get a survival ratio. Due to the plate checking interval, live cells were not observed in all wells. In cases where an empty cyst but no live cells was observed it was assumed that the germling cell had died after germination and before the plate was checked. In these cases the germination time was assigned according to the previous checking date. The initial survival time in these cases (first observation of germinated cyst, but no observation of live cell during experiment) was assigned a survival time of one, three, and six days. This range of initial survival times is used throughout the calculations made in this study.
To account for cysts that were inherently of “poor quality” (i.e., could not produce viable germlings even under illuminated conditions) and to account for non-darkness-related mortality, we scaled the dark-survival ratio to the light-survival ratio. Since the checking intervals of our light and dark plates did not match, we used the following approach: (1) the light treatment was used as a control by first fitting a model to the data (Table 1) followed by use of the derived equation to extract the light survival ratios at the specific time intervals when dark survival ratios were estimated; and (2) the dark-survival ratios were normalized against these estimated light survival ratios. A model was then fitted to the values of the scaled dark-survival ratios to get estimates of scaled dark-survival ratios for the duration of the experiment, with assumed initial survival times of germling cells of 1, 3, and 6 days.
Table 1.
Types of models evaluated for scaling of dark survival data, adjusted r2 values, and statistical significance of all parameter estimates. The difference in calculated survival ratio between the chosen first order inverse polynomial model and the other evaluated models, when assuming germination at 100 m depth.
| Type of function |
Adjusted r2 |
Significant par. estimates |
Difference from chosen function |
|
|---|---|---|---|---|
| Inverse polynomial | af=y0+(a/x) | 0.9889 | Yes | |
| f=y0+(a/x)+(b/x2) | 0.9906 | No | −0.04 | |
| Exponential decay | f=a*exp(−b*x) | 0.8404 | Yes | −0.16 |
| f=y0+a*exp(−b*x) | 0.9724 | Yes | −0.08 |
Function chosen to calculate the scaled survival ratio.
It is pertinent here to make the distinction between the viability of the cysts and of the germlings. The viability of cysts is defined as their ability to germinate, whereas the viability of germlings is defined as their capability to produce living progeny (i.e., undergo meiotic division) or to survive in the dark treatments. Darkness is known to arrest the mitotic cell cycle in the G1 phase and prohibit cell division (Taroncher-Oldenburg et al., 1997), and we assume that the lack of light has the same effect on meiotic cell division.
2.5. Water column depth-related mortality rate and comparison to model results
A comparison of our results against the existing parameterization in a model for A. fundyense population dynamics in the Gulf of Maine described by He et al. (2008) was also made. We calculated a depth-integrated mortality rate from the mortality term used in the model. The model mortality term (Mm) includes losses due to grazing, encystment, and natural cell death, and is temperature dependent. In addition, there is a loss due to the basal metabolism rate, which is part of the photosynthetic formulation of the model. In these calculations we used monthly averages from a temperature climatology (Lynch et al., 1996) in the area where the cysts were collected. In extrapolation from our experimental data to obtain a survival rate following germination and during ascent to the surface, we assume no growth in darkness. Therefore, the population experiences a net loss of cells after germination before the cells reach the illuminated surface layer where they are able to commence growth. In the calculations based on our experimental results, we used a euphotic zone depth of 30 m, representative of a low-end value for the Gulf of Maine (e.g., Hoepffner and Sathyendranath, 1992). For the calculations based on the mortality parameterization of the model, we compute the mortality vs. depth of germination based on the time required for the germlings to swim upward to the base of the euphotic zone (30 m). For the purposes of modeling germination in areas deeper than the location from which the cores were taken, the profiles were extended assuming uniform temperature from 95 m depth to 150 m. This assumption is based on the weak vertical gradients present in the 60–95 m depth interval of the climatological temperature profiles.
The proportions of cells that survive the transit from the germination depth to the euphotic zone were calculated for 5 m depth intervals for germination depths ranging from 30 to 150 m to compare our experimental results with the existing model parameterization. The surviving ratio of cells estimated from the experimental results Es is given by
| (1) |
where the aphotic time is defined by taph = (Gd − Deuph) / S, where Gd is germination depth in meters and Deuph is the depth of the euphotic zone. Swimming speed S is specified as 10 m d−1 (Bauerfeind et al., 1986; Eppley et al., 1968; Kamykowski et al., 1992). In the model, the concentration C of upward-swimming germlings in the aphotic zone is subject to mortality defined by the differential equation
| (2) |
where Mm is a temperature dependent mortality term (Mm = am × Q10 [(T−10.35)/10)], am = 0.066, and Q10 = 21.75) and Mb is the basal metabolic rate (0.2 d−1) that is included in the photosynthetic formulation of the model. The survival ratio can be computed from the solution to Eq. (2) using C = C0e−(Mm + Mb)taph using C0=1.0 at the base of the euphotic zone where taph=0.0.
Eq. (1) is the best-fit inverse first-order polynomial model of the scaled dark-survival data, where y0 and a are constants (initial survival time 1 day: y0 = 0.125, a = 0.881; initial survival time 3 days: y0 = −0.003, a = 3.010; and initial survival time 6 days: y0 = −0.202, a = 6.663). Since the method of interpolation might affect our results to some degree, we tried a suite of different approaches. Inverse-polynomial and exponential-decay functions seem to fit the data best (Table 1). Of these equations, we chose the one with the highest adjusted r2 and most significant parameter estimates. Using any of the other equations that fit the data well will cause differences in the estimated survival ratios during ascent. In Table 1, we present the difference in survival ratio from the chosen equation if any one of the other equations is used to calculate the estimated survival ratio from an arbitrary depth of 100 m.
3. Results
3.1. Cyst autofluorescence and germination rates
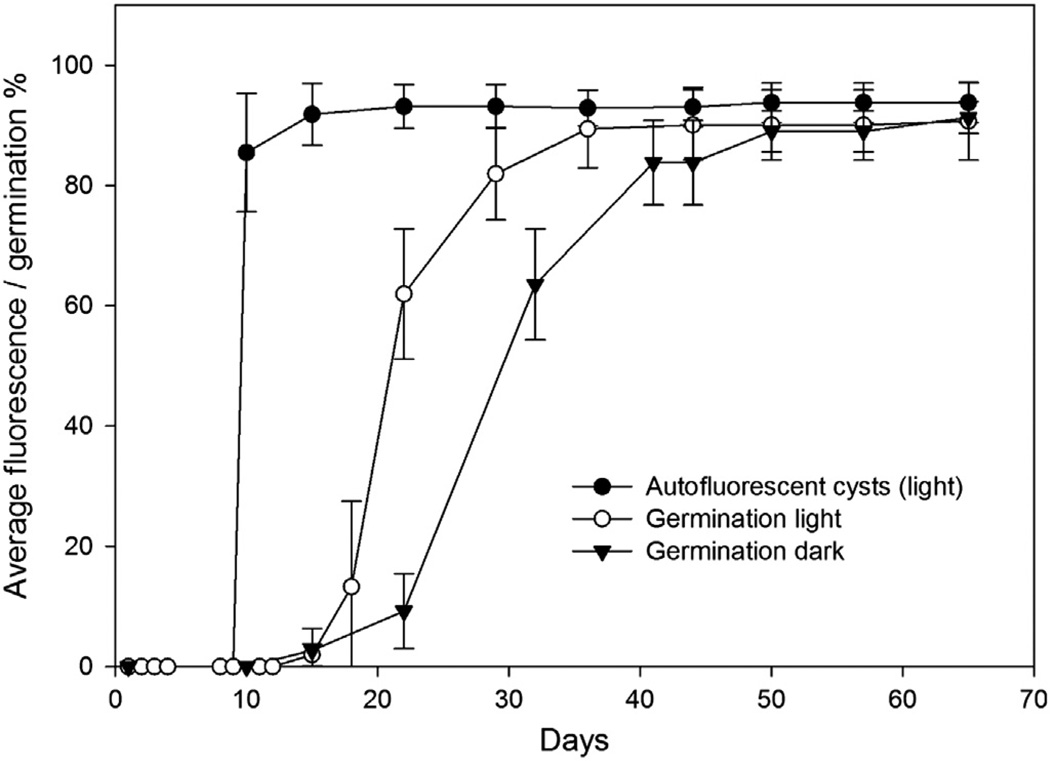
For the first 9 days, cysts incubated in the light displayed no Chl-a autofluorescence. The cysts had a small amount of granular cytoplasm and a pronounced yellow accumulation body. The first Chl-a autofluorescent cysts were observed on day 10, after which most of the cysts (up to 94%) showed autofluorescence at some stage of the experiment (Fig. 1). The development of Chl-a autofluorescence was generally synchronous, (i.e., all cysts in the light treatments started fluorescing at approximately the same time during the experiment).
Fig. 1.
Average cumulative percentage of cysts showing Chl-a autofluorescence (light treatment only) and the average cumulative percentage of cysts that germinated during the experiment. Error bars show standard deviation.
Germination was also synchronous but differed between treatments, with the dark-incubated cysts showing a 1-week lag in the germination rate maximum in comparison to the light treatment (Fig. 1). The first germinated cysts were observed on Day 15, both in the light and dark treatments. The average germination rate in the light was 0.062 d−1 (SD±0.051) and 0.038 d−1 (SD±0.029) in the dark. Both treatments reached similar excystment frequencies (91%) after approximately 50 days (Fig. 1). In the light treatments, on average 97% of cysts that showed Chl-a autofluorescence germinated during the experiment. Only one cyst that germinated was not observed to display Chl-a autofluorescence.
3.2. Germling and progeny survival
In light treatments, 47% of germinated cysts produced germlings that were able to survive and produce vegetative progeny that survived at least 7 days, i.e., a live cell was observed in the well along with an empty cyst at least once during the experiment. In the dark treatments 12% of cysts produced germlings that were able to survive for the same length of time. To account for nonviable germlings (germling mortality not related to lack of illumination), we scaled the ratio of cysts that germinated and produced a live germling cell in the dark by the ratio of cysts that were able to produce live germling cells in the light. When accounting for the non-viable germlings, 24% of cysts in the dark produced germlings that were able to survive for 7 days.
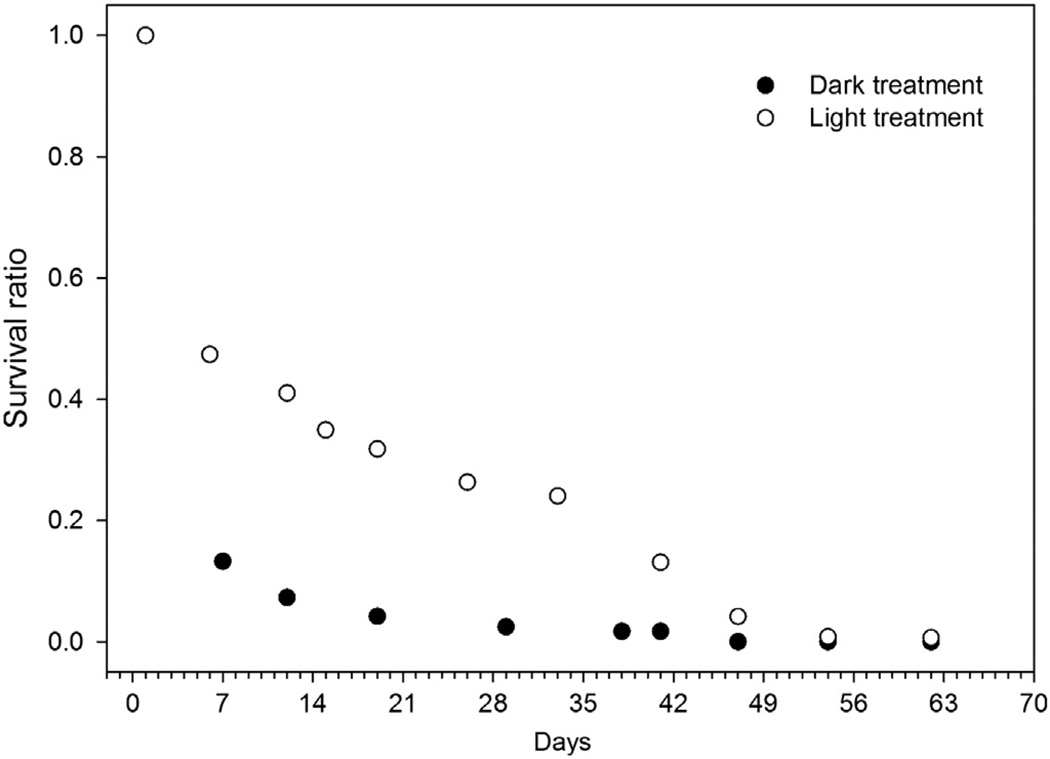
The cyst progeny experienced marked mortality during the first weeks of the experiment, even in the light treatment. After an initial high mortality, the light treatment showed a more slowly-declining survival ratio until Day 42 of the experiment, after which a more rapid decline in surviving cells again took place (Fig. 2). In subsequent calculations and model fitting to the data cut off at Day 42 was used due to this enhanced mortality in the light treatments. With the chosen model fit, the assumption of a 1 day initial survival causes the steepest initial slope of the survival curve and thus also the shortest germling half-life (Fig. 3, Table 2). The estimated half-life varies according to the chosen initial survival time, from 2.3 days to 9.5 days for the scaled dark survival results.
Fig. 2.
Survival ratios of cells in light and dark treatments during the experiment. The symbols overlay each other at the first point in time.
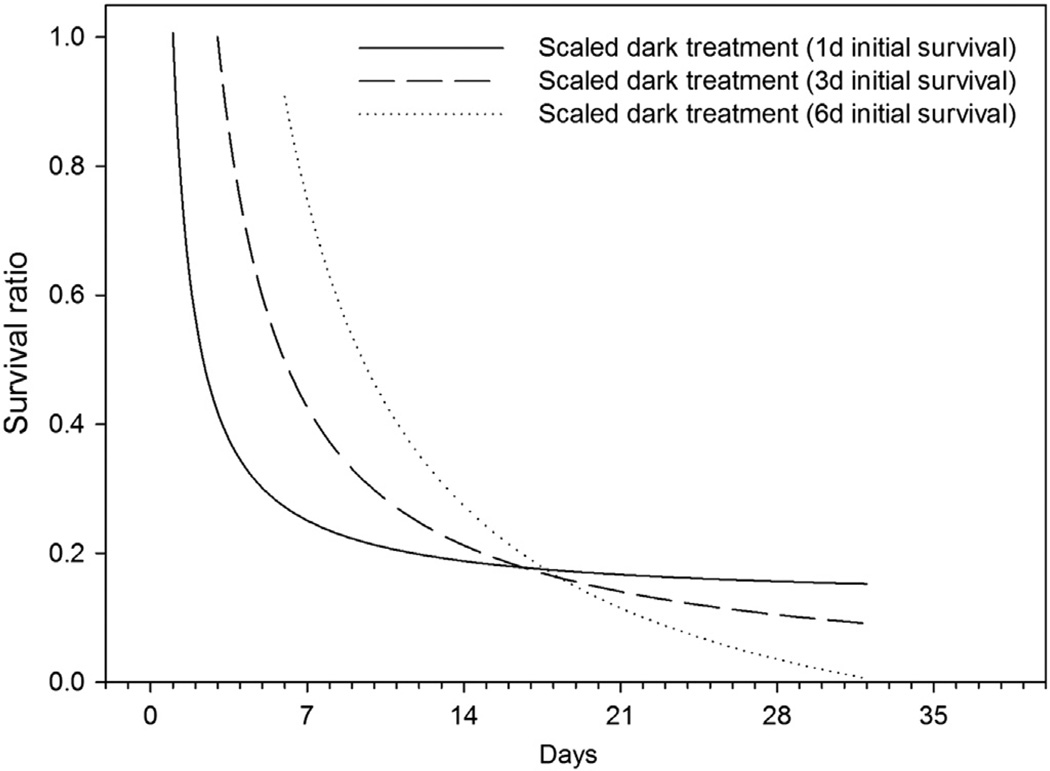
Fig. 3.
Calculated scaled dark survival ratio. The scaled survival ratio is calculated based on models (Table 1) fitted to data shown in Fig. 2 assuming initial survival times of 1, 3, and 6 days (see text for details).
Table 2.
Calculated half-life of germlings/cells in the experiment estimated based on the first degree inverse polynomial model fitted to the raw light and dark treatment data and the scaled dark treatment data. Initial survival time refers to the survival time of the germling cell before the first observation was made.
| Initial survival time (d) |
Half-life of germlings/cells (d) |
||
|---|---|---|---|
| Light treatment |
Dark treatment |
Scaled dark treatment |
|
| 1 | 3.6 | 2.0 | 2.3 |
| 3 | 8.1 | 5.3 | 6.0 |
| 6 | 11.7 | 8.6 | 9.5 |
3.3. Water-column-depth-related mortality rate and comparison to model results
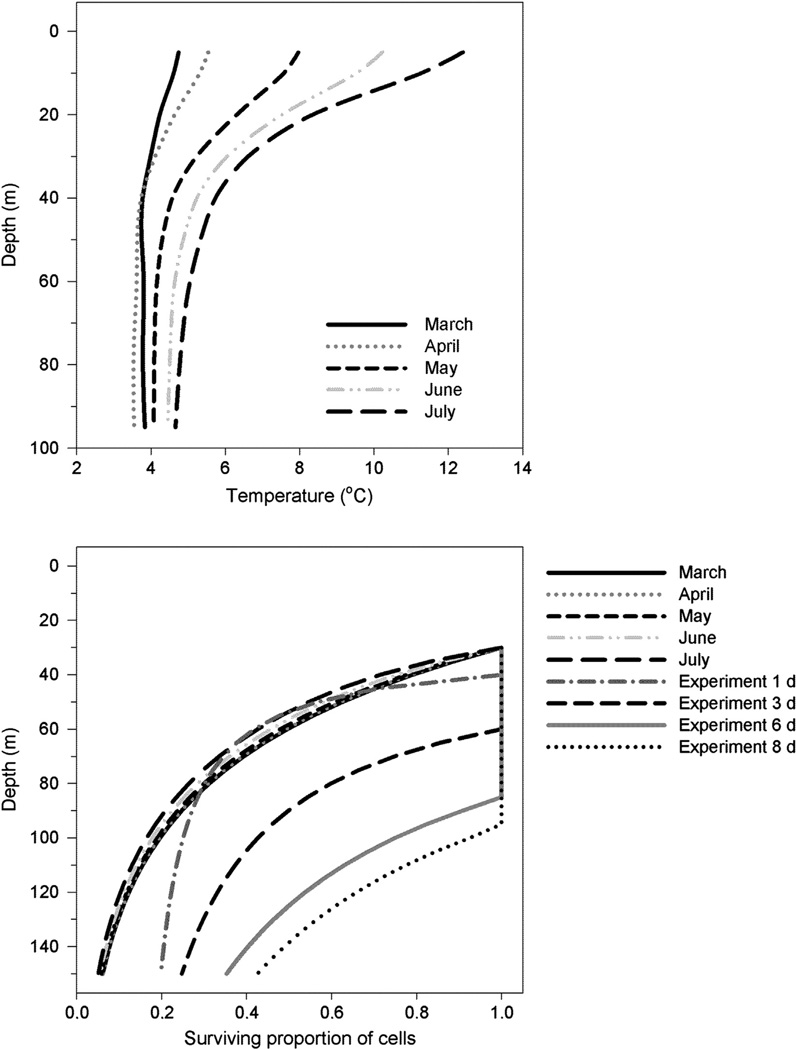
The surviving proportion of cells from hypothetical cyst seedbeds lying at 30–150 m depth experiencing mortality due to darkness was estimated assuming the initial survival times of germling cells to be 1, 3, and 6 days. The scaled dark survival rates are shown in Fig. 3. These survival times are reasonable, taking into account the depth of cyst seedbeds, the depth of the euphotic zone, and the average swimming speed of dinoflagellates. These results are compared against the mortality term used in a coupled physical–biological model describing the population dynamics of A. fundyense in the Gulf of Maine (Fig. 4). The calculations do not include growth and only describe the mortality and respiration of the initial germling inoculum population. Based on our results approximately 25–75% (93% if an initial survival time of 8 days is assumed) of the cells germinating at a depth of 100 m could survive the transit to the euphotic zone, whereas the model allows for 17–20% of the cells to reach the surface layer (Fig. 4).
Fig. 4.
Temperature climatology used for model calculations of the survival of cells migrating upward from a specific depth at an average swimming speed of 10 m d−1 (upper panel); and comparison of the survival ratio of cells germinating at different water column depths between our experimental scaled dark treatment values (denoted experiment 1–8 d) and the model parameterization (lower panel) used by He et al. (2008), (denoted March–July); see text for details. Calculations were done with a mean euphotic zone depth of 30 m representing the natural variation found in the Gulf of Maine.
4. Discussion
Information on the fate and survival potential of newly-germinated cells from resting cysts in bottom sediments is critical in the study of population dynamics of cyst-forming HAB species such as A. fundyense. Here we conducted laboratory experiments to characterize the germination and survival rates of cysts and cells incubated in the light vs. the dark. We also compared our measured survival rates to the rates used in the physical–biological model used to simulate A. fundyense population dynamics in the Gulf of Maine. Our results show a marked initial mortality of germling cells in both light and dark treatments, prompting a reevaluation of how we think about cyst viability. In addition to the potential to germinate, we need to account for the ability of the germling to divide and produce viable vegetative cells. Here we provide direct measurements of survival of germling cells and their vegetative progeny for A. fundyense cysts from the Gulf of Maine, specifically looking at the effect of darkness on the survival of germling cells. Our aim is to improve existing indirect estimates of the emergence of germinated cells from coastal sediments and their role in bloom initiation.
4.1. Chl-a autofluorescence and germination rates
The increase in Chl-a fluorescence and germination of cysts were synchronous in the experiment. Synchronous development of Chl-a autofluorescence, and also germination, has been previously observed (Anderson and Keafer, 1985; Anderson et al., 2005; Genovesi et al., 2009), even though regional differences exist. In our study 94% of the cysts showed autofluorescence at some point during the experiment (light treatment) and of these, 97% germinated at some point during the experiment. We assume that a similar proportion of dark-treatment cysts showed auto-fluorescence prior to germination. If the estimate holds true, 94% of cysts in the dark that would have shown Chl-a autofluorescence would have germinated. Our observed germination rates are in line with previous estimates (Anderson et al., 2005). We can conclude that cyst viability in our study was high, and in line with previous studies (Anderson et al., 2005).
Anderson et al. (2005) developed an indirect method for estimating the flux of cells germinated from deep coastal sediments. Briefly, the flux was estimated by a combination of laboratory measurements of cyst autofluorescence and germination, and observations of cyst autofluorescence in the field. A sediment slurry containing a natural high concentration of cysts was made up of one part of natural sediment and seven parts of f/2 medium. Aliquots of this slurry were incubated in a range of temperatures, as well as under illumination and in darkness. The number of cysts and the fraction of Chl-a autofluorescent cysts were counted at specific time intervals. The difference between the initial cyst number and the cyst number after incubation was used to calculate the percentage of germinated cysts. This approach was implemented in the physical–biological model used for studies of A. fundyense bloom dynamics in the Gulf of Maine (McGillicuddy et al., 2005; Stock et al., 2005; He et al., 2008). However, this method assumes survival of all germling cells, which is an aspect that has not received much attention in the past. Genovesi et al. (2009) made observations on germling cell viability for shallow water A. tamarense populations and concluded that viability was considerably lower than the germination success would have indicated, with approximately 27% of cysts producing germling cells that were able to survive and subsequently divide. In our experiment, 47% of cysts incubated under illuminated conditions produced live germling cells that were able to divide and survive at least seven days. For the dark-incubated cysts, the percentage of germling cells that survived for seven days was 12%. These results may be indicative of the quality of the cysts (e.g., the amount of stored reserves relative to what is needed to support the metabolic demands of germination, motility, and cell division—see below). As such, studies on the spatial or temporal variation of cyst viability and germling survival would be informative.
Our results show that darkness retards germination timing, as has been previously observed for Alexandrium and other dinoflagellate genera (Anderson et al., 1987; Bravo and Anderson, 1994). We observed a similar germination frequency in both light and dark treatments after 50 days, indicating no light requirement for germination. Genovesi et al., 2009 noted that total darkness depressed the cumulative excystment frequency of A. tamarense somewhat (62%) compared to treatments that had received light exposures (100 µmol photons m−2 s−1) of 1, 3, 6, and 12 h, before dark incubation (average excystment frequency 80%). However, the cysts in that particular study were harvested from a shallow sea area, which could cause effective selection on the population favoring cysts that have light-inducible excystment.
4.2. Survival of germlings and non-darkness related mortality
In our experiment, only a fraction of cysts that germinated seemed to be able to produce viable germlings. This can be due to the fact that we used sediment from the 1 to 3 cm layer from the cores, which would render the cysts slightly older on average than those at the surface (Keafer et al., 1992), and also potentially affect their quality (Genovesi et al., 2009). Older cysts of poorer quality, i. e., they have used more of their storage energy during prolonged quiescence, could produce less-viable progeny, especially if germination occurs in the dark where synthesis of new energy stores is not possible. The cysts would be able to germinate but the germling cells would not be able to divide or the cells would die soon thereafter. The length of cold dark storage has been noted to affect cyst quality, with longer storage times causing a decrease in the ability of cysts to produce viable germlings (Genovesi et al., 2009). Darkness is also known to cause the cell cycle to be arrested in the G1 phase and inhibit mitotic cell division (Taroncher-Oldenburg et al., 1997). If darkness affects meiotic cell division in a similar manner, this effectively means that all cells germinating at aphotic depths remain in the germling stage until they reach photic depths, and the cell cycle is able to resume. How this would affect cell survival is not known.
The cells in our experiment were affected by other mortality factors besides those caused by darkness directly, as seen from the declining survival ratio in the light treatments. The survival of cells also declined rapidly after Day 42 of the experiment for some unknown reason. The most plausible causes that can be evoked for premature cell death are poor cyst quality, producing non-viable germlings, and later on in the experiment, density-dependent mortality in the light treatment. However, it could also be other factors related to the incubation conditions. Therefore, we need to account for mortality in the dark treatments that is not caused by darkness. For this, the light treatment can function as a control.
In this regard, the simplest approach is to scale the survival ratio of germlings in the dark to the survival ratio of germlings in the light. This scaling was done by first fitting a model to the light treatment data, to take into account a range of different initial survival times for the germling cells and to synchronize the lightand dark-treatment observations. The results from the model fit were used to calculate the scaled dark survival, by dividing the dark-treatment survival ratio by the light-treatment survival ratio. These scaled results were used when we compared our results to model mortality parameterizations. We make the assumption that the same factors (beside darkness) are causing mortality in both the light and dark treatment. It might even be possible that the illumination itself is causing mortality in the light treatments; however, that seems unlikely since the light level we used, 250 µmol photons m−2 s−1, falls in the interval used to culture A. fundyense, 200–400 µmol photons m−2 s−1 (D. Kulis, Personal Communication).
Because of the lack of frequent observations, the assumption of initial survival time of germlings of 1, 3, and 6 days cause a marked variation in the estimated half-lives of the germlings and cells. The difference in half-life between the 1- and 6-day initial survival time assumption scenarios for the scaled dark treatment is 7.2 day (Table 2). However, a previous study has shown that germlings of poor quality do not survive for long (Genovesi et al., 2009). Only 27% of the cysts in that study produced germlings that lived longer than 1 day. Thus, rather short initial survival times seem a more plausible assumption than longer ones. These results argue for experiments with more frequent observations of germling survival in the dark for coastal A. fundyense populations.
4.3. Survival during ascent: Model vs. data comparisons
In order to estimate the effects of darkness on the survival of germlings and their progeny in natural waters, we calculated the survival of germinated cells moving upward through a deep water column. We also compared these results to parameterizations from an existing coupled physical–biological model used to simulate the population dynamics of A. fundyense in the Gulf of Maine (Fig. 4). The model assumes that 100% of the cysts in the top 1 cm of surface sediments can germinate, with the survival of the germling cells and their progeny determined by a temperature-dependent mortality term and a basal metabolism loss term (He et al., 2008). This mortality term allows 17–20% of the population directly produced from germinated cysts to reach the bottom of the illuminated surface layer at a 30 m depth, in a 100 m water column overlying bottom sediments where the germination occurs.
Dinoflagellate cells have been reported to reach swimming speeds of 5–15 m d−1 (Bauerfeind et al., 1986; Eppley et al., 1968; Kamykowski et al., 1992). Without any effect from hydrodynamic transport, a cell germinating on the bottom at 100 m depth and swimming at an average speed of 10 m d−1 would require approximately 10 days to reach the surface, and seven days to reach the euphotic zone where photosynthesis and cell division can commence, assuming a euphotic layer depth of approximately 30 m for the Gulf of Maine (Hoepffner and Sathyendranath, 1992). The spatial distribution of cysts and cyst germination appear to be an important part of bloom dynamics, as indicated by coupled physical–biological models (McGillicuddy et al., 2005; Stock et al., 2005; He et al., 2008; Li et al., 2009; McGillicuddy et al., 2011; Anderson et al., 2014). Most of the cysts lie at depths below 100 m depth, either in bottom sediments (Anderson et al., 2005; Anderson et al., 2014), or in a near-bottom benthic nepheloid layer (Kirn et al., 2005; Pilskaln et al., 2014). Therefore, it is important to evaluate how large a proportion of cysts actually produce living progeny that survive long enough to further divide and contribute to population growth in illuminated water layers, assuming that the cells do not grow in the dark (Taroncher-Oldenburg et al., 1997).
Our study showed that germling survival is lower in the dark, which is the condition that prevails in deep waters below the euphotic zone. In darkness, the half-life of germling cells is on average approximately 30–35% shorter than in the light. Depending on the assumption of initial survival time (1, 3, or 6 d) for germling cells, 27–35%, 50–75% or 90–100% of cells would survive a transit to 30 m depth from bottom depths ranging from 90 to 70 m, respectively. This percentage of surviving cells is subject to additional mortality due to grazing and other factors, which are not considered in this study. The chosen model fit to our data obviously affects the results of this comparison. The curves for the three different initial survival-time estimates start converging when approaching 17 days (Fig. 3). After this, survival becomes the opposite for the one and six day initial survival time estimates. In our calculations the longest time span is 12 days. The curves do not converge; however, they approach each other, which can be seen as a decreasing difference in survival ratio when germination depth increases (Figs. 3 and 4). Different model fits will behave slightly differently and give somewhat different results, e.g., a single, one-parameter exponential decay model will yield a survival ratio that is 0.16 units smaller than what the best-fit model yields (Table 1). What is important though is the fact that not all cysts are able to produce germlings that can survive the ascent to the illuminated surface layer and that most of the mortality takes place during the first several days after germination. This indicates that caution should be exercised when evaluating the quantitative impact of cyst seed banks on bloom formation based solely on cyst concentrations in the surface sediments. Survival ratios are affected by cyst quality, so both bottom depth and the average age or condition of cysts in the seed bank will affect the ability of the cysts to seed blooms effectively.
Our data are obviously compromised by the fact that the experiments were carried out in the laboratory. The plate-checking interval also presents an important caveat to the interpretation of the data. Survival of cells during the first 10 days of our experiment is the most important time interval with regard to the model comparisons. During this interval we have only a single observation. By changing the length of time of initial survival to 1, 3, or 6 days we can markedly affect the results. If 100% of the cells would have survived for 6 days during the unchecked interval, the calculations indicate that 100% of germinated cells would make it to the euphotic zone from a bottom depth of approximately 85 m. Germling viability and the initial survival time of germling cells therefore need to be further investigated in order to make our estimates more accurate. However, the relative differences in initial survival between light and dark treatments (47% vs. 12% of cysts producing germlings that were able to divide in 7 days) should prompt a more accurate description of germling emergence, survival, and vertical movement of these cells in models. Model parameterizations might be underestimating the impact of deep cyst seed beds on bloom formation. An effort should be made to more accurately estimate the proportion of germlings and their progeny that reach the illuminated layers of the water column, in relation to the number of germinated cysts.
Acknowledgments
E. Vahtera was funded by the Academy of Finland (Grant no. 130934) and B. Gomez-Crespo was supported by a Xunta de Galicia Ángeles Alvariño fellowship. Additional funding support was also provided by the National Oceanic Atmospheric Administration ECOHAB program through grants NA06NOS4780245 and NA09NOS4780193, and from National Science Foundation grants OCE-0430724, OCE-0911031, OCE-1314642 and National Institute of Environmental Health Sciences grants 1P50-ES01274201 and 1P01ES021923-01 through the Woods Hole Center for Oceans and Human Health. We are also grateful for technical assistance from Z. Bonin, B. Keafer, K. Smith, and D. Kulis. R. He, and Y. Li are acknowledged for their valuable comments on the manuscript. This is ECOHAB contribution number 734.
References
- Anderson DM. Effects of temperature conditioning on development and germination of Gonyaulax tamarensis (Dinophyceae) hypnozygote. J. Phycol. 1980;16:166–172. [Google Scholar]
- Anderson DM, Keafer BA. Dinoflagellate cyst dynamics in coastal and estuarine waters. In: Anderson DM, White AW, Baden DG, editors. Toxic Dinoflagellates, Proceedings of the Third International Conference. New York: Elsevier; 1985. pp. 219–224. [Google Scholar]
- Anderson DM, Lively JJ, Reardon EM, Price CA. Sinking characteristics of dinoflagellate cysts. Limnol. Oceanogr. 1985;30:1000–1009. [Google Scholar]
- Anderson DM, Keafer BA. An endogenous annual clock in the toxic marine dinoflagellate Gonyaulax tamarensis. Nature. 1987;325:616–617. doi: 10.1038/325616a0. [DOI] [PubMed] [Google Scholar]
- Anderson DM, Taylor CD, Armbrust EV. The effects of darkness and anaerobiosis on dinoflagellate cyst germination. Limnol. Oceanogr. 1987;32:340–351. [Google Scholar]
- Anderson DM, Kulis DM, Doucette GJ, Gallager JC, Balech E. Biogeography of toxic dinoflagellates in the genus Alexandrium from the northeast United States and Canada as determined by morphology, bioluminescence, toxin composition, and mating compatibility. Mar. Biol. 1994;120:467–478. [Google Scholar]
- Anderson DM. Physiology and bloom dynamics of toxic Alexandrium species, with emphasis on life cycle transitions. In: Anderson DM, Cembella AD, Hallegraeff GM, editors. The Physiological Ecology of Harmful Algal Blooms. Heidelberg: Springer-Verlag; 1998. pp. 29–48. [Google Scholar]
- Anderson DM, Fukuyo Y, Matsuoka M. Cyst methodologies. In: Hallegraef GM, Anderson DM, Cembella AD, editors. Manual on Harmful Marine Microalgae. Monographs on Oceanographic Methodology 11. Paris: UNESCO; 2003. pp. 165–190. [Google Scholar]
- Anderson DM, Stock CA, Keafer BA, Bronzino Nelson A, Thompson B, McGillicuddy DJ, Jr, Keller M, Matrai PA, Martin J. Alexandrium fundyense cyst dynamics in the Gulf of Maine. Deep Sea Res. II. 2005;52(Pt. II):2522–2542. [Google Scholar]
- Anderson DM, Keafer BA, Kleindinst JL, McGillicuddy DJ, Martin JL, Norton K, Pilskaln CH, Smith JL, Sherwood CR, Butman B. Alexandrium fundyense cysts in the Gulf of Maine: long-term time series of abundance and distribution, and linkages to past and future blooms. Deep Sea Res. II. 2014;103:6–26. doi: 10.1016/j.dsr2.2013.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauerfeind E, Elbrachter M, Steiner R, Throndsen J. Application of Laser Doppler Spectroscopy (LDS) in determining swimming velocities in motile phytoplankton. Mar. Biol. 1986;93(3):323–327. [Google Scholar]
- Bravo I, Anderson DM. The effects of temperature, growth medium and darkness on excystment and growth of the toxic dinoflagellate Gymnodinium catenatum from north-west Spain. J. Plankton Res. 1994;16:513–525. [Google Scholar]
- Craib JS. A sampler for taking short undisturbed marine cores. J. Cons. 1965;30:34–39. [Google Scholar]
- Dale B. Dinoflagellate resting cysts: ‘benthic plankton'. In: Fryxell GA, editor. Survival Strategies of Algae. Cambridge: Cambridge University Press; 1983. pp. 69–136. [Google Scholar]
- Eppley RW, Holm-Hansen O, Strickland JD. Some observations on the vertical migration of dinoflagellates. J. Phycol. 1968;4:333–340. doi: 10.1111/j.1529-8817.1968.tb04704.x. [DOI] [PubMed] [Google Scholar]
- Genovesi B, Laabir M, Masseret E, Collos Y, Vaquer A, Grzebyk D. Dormancy and germination features in resting cysts of Alexandrium tamarense species complex (Dinophyceae) can facilitate bloom formation in a shallow lagoon (Thau, southern France) J. Plankton Res. 2009;31:1209–1224. [Google Scholar]
- He R, McGillicuddy DJ, Jr, Keafer BA, Anderson DM. Historic 2005 toxic bloom of Alexandrium fundyense in the Western Gulf of Maine: 2. Coupled biophysical numerical modeling. J. Geophys. Res. 2008;113:C07040. doi: 10.1029/2007JC004602. , http://dx.doi.org/10.1029/2007JC004602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoepffner N, Sathyendranath S. Bio-optical characteristics of coastal waters: absorption spectra of phytoplankton and pigment distribution in the western North Atlantic. Limnol. Oceanogr. 1992;37(8):1660–1679. [Google Scholar]
- Kamykowski D, Reed RE, Kirkpatrick GJ. Comparison of the sinking velocity, swimming velocity, rotation and path characteristics among six marine dinoflagellate species. Mar. Biol. 1992;113(2):319–328. [Google Scholar]
- Keafer BA, Buesseler KO, Anderson DM. Burial of living dinoflagellate cysts in estuarine and nearshore sediments. Mar. Micropaleontol. 1992;20:147–161. [Google Scholar]
- Kirn SL, Townsend DW, Pettigrew NR. Suspended Alexandrium spp. hypnozygote cysts in the Gulf of Maine. Deep Sea Res. II. 2005;52:2543–2559. [Google Scholar]
- Kremp A, Anderson DM. Factors regulating germination of resting cysts of the spring bloom dinoflagellate Scripsiella hangoei from the northern Baltic Sea. J. Plankton Res. 2000;22(7):1311–1327. [Google Scholar]
- Li Y, He R, McGillicuddy DJ, Jr, Anderson DM, Keafer BA. Investigation of the 2006 Alexandrium fundyense bloom in the Gulf of Maine: in-situ observations and numerical modeling. Cont. Shelf Res. 2009;29:2069–2082. doi: 10.1016/j.csr.2009.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch DR, Ip JTC, Naimie CE, Werner FE. Comprehensive coastal circulation model with application to the Gulf of Maine. Cont. Shelf Res. 1996;16:875–906. [Google Scholar]
- Matrai P, Thompson B, Keller M. Circannual excystment of resting cysts of Alexandrium spp. from eastern Gulf of Maine populations. Deep Sea Res. II. 2005a;52:2560–2568. [Google Scholar]
- Matsuoka K, Fukuyo Y. Taxonomy of cysts. In: Hallegraef GM, Anderson DM, Cembella AD, editors. Manual on Harmful Marine Microalgae. Paris: UNESCO; 2003. pp. 563–592. [Google Scholar]
- McGillicuddy DJ, Anderson DM, Lynch D, Townsend D. Mechanisms regulating large-scale seasonal fluctuations in Alexandrium fundyense populations in the Gulf of Maine: results from a physical–biological model. Deep Sea Res. II. 2005;52:2698–2714. [Google Scholar]
- McGillicuddy DJ, Townsend DW, He R, Keafer BA, Kleindinst JL, Li Y, Manning JP, Mountain DG, Thomas MA, Anderson DM. Suppression of the 2010 Alexandrium fundyense bloom by changes in physical, biological, and chemical properties of the Gulf of Maine. Limnology and Oceanography. 2011;56:2411–2426. doi: 10.4319/lo.2011.56.6.2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez CC, Roy S, Levasseur M, Anderson DM. Control of Alexandrium tamarense cysts from the lower St Lawrence Estuary Canada. J. Phycol. 1998;34(2):242–249. [Google Scholar]
- Pilskaln CH, Hayashi K, Keafer BA, Anderson DM, McGillicuddy DJ., Jr Benthic nepheloid layers in teh Gulf of Maine and Alexandrium cyst inventories. Deep Sea Res. II. 2014;103:55–65. doi: 10.1016/j.dsr2.2013.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rengefors K, Anderson DM. Environmental and endogenous regulation of cyst germination in two freshwater dinoflagellates. J. Phycol. 1998;34(4):568–577. [Google Scholar]
- Schwinghamer P, Anderson DM, Kulis DM. Separation and concentration of living dinoflagellate resting cysts from marine sediments via density gradient centrifugation. Limnol. Oceanogr. 1991;36:588–592. [Google Scholar]
- Stock CA, McGillicuddy DJ, Jr, Solow AR, Anderson DM. Evaluating hypotheses for the initiation and development of Alexandrium fundyense blooms in the western Gulf of Maine using a coupled physical–biological model. Deep Sea Res. II. 2005;52:2715–2744. [Google Scholar]
- Taroncher-Oldenburg G, Kulis DM, Anderson DM. Toxin variability during the cell cycle of the dinoflagellate Alexandrium fundyense. Limnol. Oceanogr. 1997;42(5, Pt. 2):1178–1188. [Google Scholar]
- Wall D. Biological problems concerning fossilizable dinoflagellates. Geosci. Man III. 1971:1–15. [Google Scholar]