Figure 4.
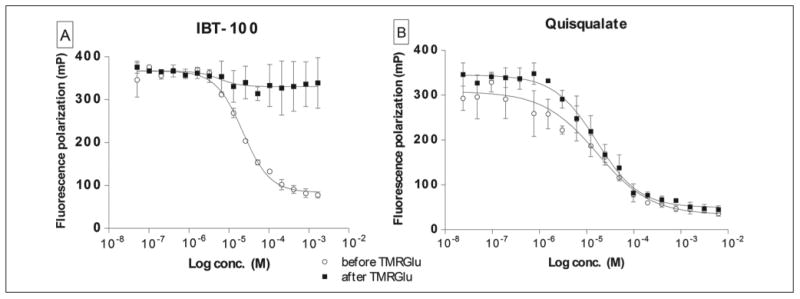
Fluorescence polarization (FP) assay allows distinguishing between the noncompetitive/allosteric (A) and competitive (B) mode of inhibition. Both noncompetitive and competitive inhibitors block the formation of the glutamate carboxypeptidase II (GCPII)/TMR-X-Lys-urea-Glu (TMRGlu), when the inhibitor is preincubated with the enzyme prior to the probe addition (empty circles; IC50 = 22 μM and IC50 = 46 μM for IBT-100 and quisqualate, respectively). A noncompetitive inhibitor, however, is unable to displace the probe when added to the preformed GCPII/TMRGlu complex (panel A, squares), whereas the addition of a competitive inhibitor to the preformed GCPII/TMRGlu complex is accompanied by a decrease in the FP value (panel B, squares; IC50 = 12 μM) as a result of the probe displacement by the inhibitor.
