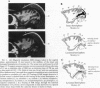
Abstract
Blindsight is a phenomenon in which human patients with damage to striate cortex deny any visual sensation in the resultant visual field defect but can nonetheless detect and localize stimuli when persuaded to guess. Although monkeys with striate lesions have also been shown to exhibit some residual vision, it is not yet clear to what extent the residual capacities in monkeys parallel the phenomenon of human blindsight. To clarify this issue, we trained two monkeys with unilateral lesions of striate cortex to make saccadic eye movements to visual targets in both hemifields under two conditions. In the condition analogous to clinical perimetry, they failed to initiate saccades to targets presented in the contralateral hemifield and thus appeared "blind." Only in the condition where the fixation point was turned off simultaneously with the onset of the target--signaling the animal to respond at the appropriate time--were monkeys able to localize targets contralateral to the striate lesion. These results indicate that the conditions under which residual vision is demonstrable are similar for monkeys with striate cortex damage and humans with blindsight.
Full text
PDF
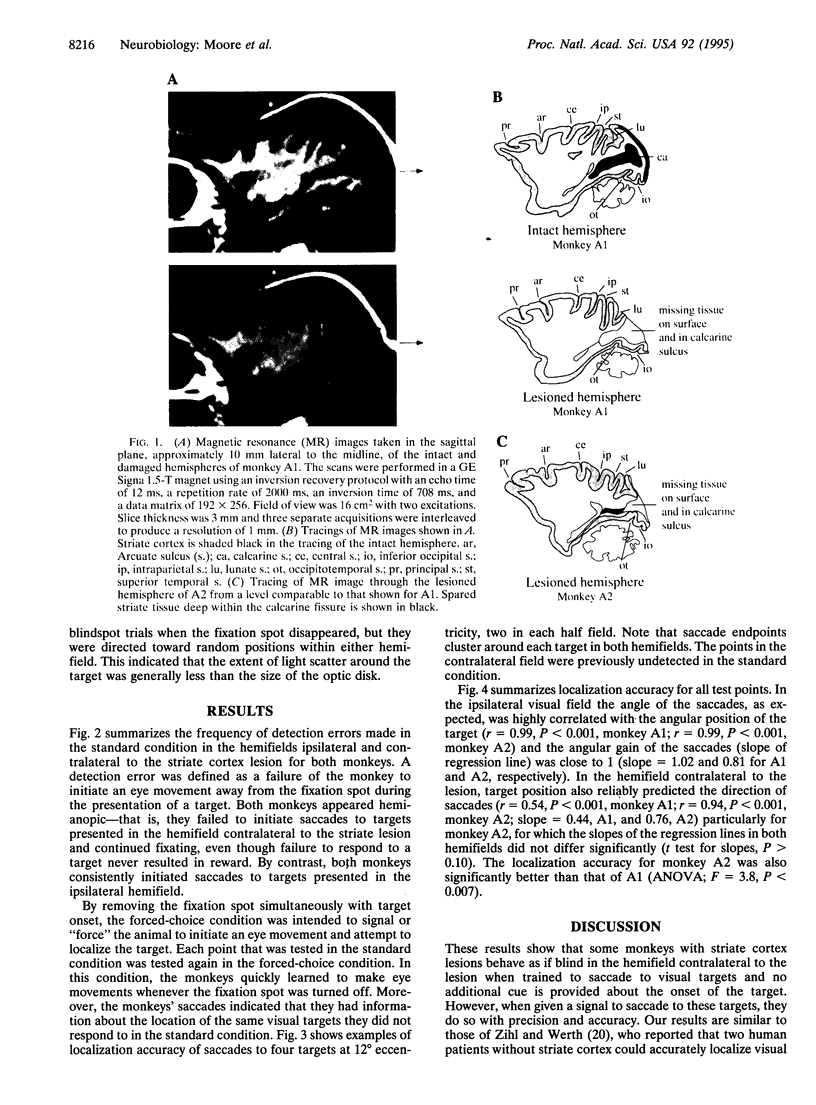
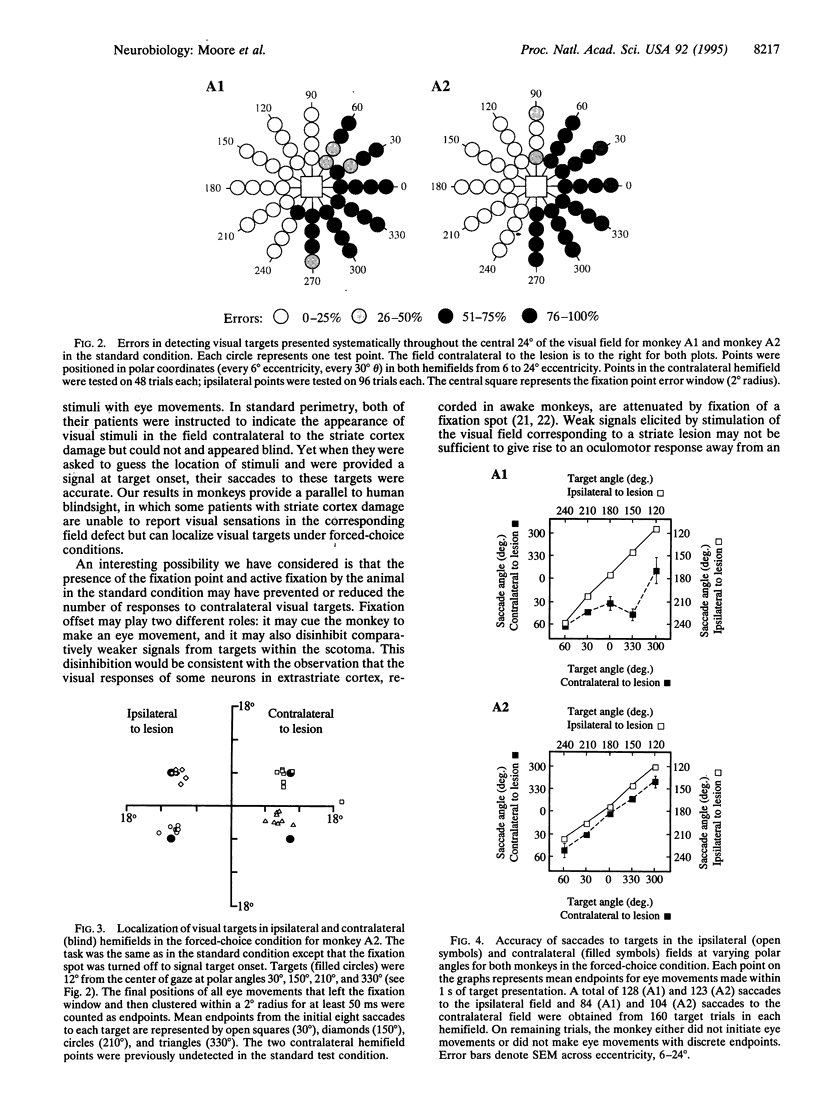

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blythe I. M., Kennard C., Ruddock K. H. Residual vision in patients with retrogeniculate lesions of the visual pathways. Brain. 1987 Aug;110(Pt 4):887–905. doi: 10.1093/brain/110.4.887. [DOI] [PubMed] [Google Scholar]
- Cowey A. Perimetric study of field defects in monkeys after cortical and retinal ablations. Q J Exp Psychol. 1967 Aug;19(3):232–245. doi: 10.1080/14640746708400098. [DOI] [PubMed] [Google Scholar]
- Cowey A., Stoerig P. Blindsight in monkeys. Nature. 1995 Jan 19;373(6511):247–249. doi: 10.1038/373247a0. [DOI] [PubMed] [Google Scholar]
- Cowey A., Stoerig P., Perry V. H. Transneuronal retrograde degeneration of retinal ganglion cells after damage to striate cortex in macaque monkeys: selective loss of P beta cells. Neuroscience. 1989;29(1):65–80. doi: 10.1016/0306-4522(89)90333-3. [DOI] [PubMed] [Google Scholar]
- Cowey A., Stoerig P. The neurobiology of blindsight. Trends Neurosci. 1991 Apr;14(4):140–145. doi: 10.1016/0166-2236(91)90085-9. [DOI] [PubMed] [Google Scholar]
- Dineen J., Hendrickson A., Keating E. G. Alterations of retinal inputs following striate cortex removal in adult monkey. Exp Brain Res. 1982;47(3):446–456. doi: 10.1007/BF00239362. [DOI] [PubMed] [Google Scholar]
- Girard P., Salin P. A., Bullier J. Response selectivity of neurons in area MT of the macaque monkey during reversible inactivation of area V1. J Neurophysiol. 1992 Jun;67(6):1437–1446. doi: 10.1152/jn.1992.67.6.1437. [DOI] [PubMed] [Google Scholar]
- Gross C. G. Contribution of striate cortex and the superior colliculus to visual function in area MT, the superior temporal polysensory area and the inferior temporal cortex. Neuropsychologia. 1991;29(6):497–515. doi: 10.1016/0028-3932(91)90007-u. [DOI] [PubMed] [Google Scholar]
- Judge S. J., Richmond B. J., Chu F. C. Implantation of magnetic search coils for measurement of eye position: an improved method. Vision Res. 1980;20(6):535–538. doi: 10.1016/0042-6989(80)90128-5. [DOI] [PubMed] [Google Scholar]
- Mohler C. W., Wurtz R. H. Role of striate cortex and superior colliculus in visual guidance of saccadic eye movements in monkeys. J Neurophysiol. 1977 Jan;40(1):74–94. doi: 10.1152/jn.1977.40.1.74. [DOI] [PubMed] [Google Scholar]
- Newsome W. T., Wurtz R. H., Dürsteler M. R., Mikami A. Punctate chemical lesions of striate cortex in the macaque monkey: effect on visually guided saccades. Exp Brain Res. 1985;58(2):392–399. doi: 10.1007/BF00235320. [DOI] [PubMed] [Google Scholar]
- Perenin M. T., Jeannerod M. Visual function within the hemianopic field following early cerebral hemidecortication in man--I. Spatial localization. Neuropsychologia. 1978;16(1):1–13. doi: 10.1016/0028-3932(78)90037-4. [DOI] [PubMed] [Google Scholar]
- Poppel E., Held R., Frost D. Leter: Residual visual function after brain wounds involving the central visual pathways in man. Nature. 1973 Jun 1;243(5405):295–296. doi: 10.1038/243295a0. [DOI] [PubMed] [Google Scholar]
- Richmond B. J., Wurtz R. H., Sato T. Visual responses of inferior temporal neurons in awake rhesus monkey. J Neurophysiol. 1983 Dec;50(6):1415–1432. doi: 10.1152/jn.1983.50.6.1415. [DOI] [PubMed] [Google Scholar]
- Rodman H. R., Gross C. G., Albright T. D. Afferent basis of visual response properties in area MT of the macaque. I. Effects of striate cortex removal. J Neurosci. 1989 Jun;9(6):2033–2050. doi: 10.1523/JNEUROSCI.09-06-02033.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders M. D., Warrington E. K., Marshall J., Wieskrantz L. "Blindsight": Vision in a field defect. Lancet. 1974 Apr 20;1(7860):707–708. doi: 10.1016/s0140-6736(74)92907-9. [DOI] [PubMed] [Google Scholar]
- Segraves M. A., Goldberg M. E., Deng S. Y., Bruce C. J., Ungerleider L. G., Mishkin M. The role of striate cortex in the guidance of eye movements in the monkey. J Neurosci. 1987 Oct;7(10):3040–3058. doi: 10.1523/JNEUROSCI.07-10-03040.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weller R. E., Kaas J. H. Parameters affecting the loss of ganglion cells of the retina following ablations of striate cortex in primates. Vis Neurosci. 1989 Oct;3(4):327–349. doi: 10.1017/s0952523800005514. [DOI] [PubMed] [Google Scholar]
- Zihl J., Werth R. Contributions to the study of "blindsight"--I. Can stray light account for saccadic localization in patients with postgeniculate field defects? Neuropsychologia. 1984;22(1):1–11. doi: 10.1016/0028-3932(84)90002-2. [DOI] [PubMed] [Google Scholar]