Fig. 1.
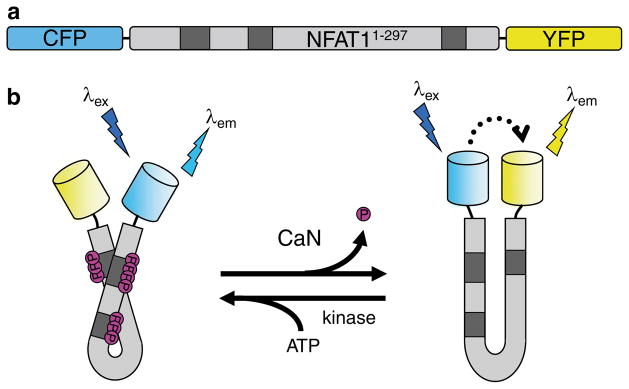
The design of CaNAR. (a) CaNAR features the N-terminal 297 amino acids of NFAT1 sandwiched between the FRET donor CFP and the FRET acceptor YFP. Shaded boxes indicate the sites where NFAT is constitutively phosphorylated. (b) Dephosphorylation of the NFAT N-terminal domain by CaN results in a conformational change that exposes an NLS. In CaNAR, this serves as a molecular switch that induces a change in the proximity and relative orientation of CFP and YFP, leading to a FRET change (dotted arrow). This is reversed upon rephosphorylation of the reporter by cellular kinases
