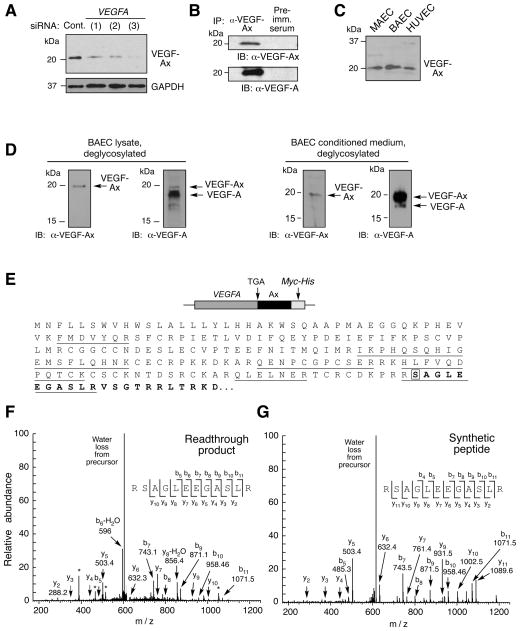
Figure 2. VEGF-Ax is a Novel Isoform of VEGFA Generated by Translational Readthrough.
(A) VEGFA-specific siRNAs inhibit VEGF-Ax expression. Bovine ECs were transfected with three different VEGFA-specific siRNAs and VEGF-Ax in cell lysates determined by immunoblot with anti-VEGF-Ax antibody.
(B) VEGF-Ax is an authentic VEGF-A isoform. Lysates from bovine EC were immunoprecipitated with anti-VEGF-Ax antibody or pre-immune (Pre-imm.) serum and subjected to immunoblot analysis with anti-VEGF-Ax and anti-VEGF-A antibodies.
(C) VEGF-Ax is expressed by murine aortic ECs (MAEC), bovine aortic ECs (BAEC), and human umbilical vein ECs (HUVEC). Cell lysates were subjected to immunoblot analysis with anti-VEGF-Ax antibody.
(D) Separation of VEGF-Ax and VEGF-A isoforms in EC. BAEC lysates (left) and conditioned media (right) were deglycosylated and resolved on 16% Tricine gel before immunoblot analysis with anti-VEGF-Ax and anti-VEGF-A antibodies.
(E) Amino acid sequence of VEGF-Ax. Peptides identified by mass spectrometry (underline), readthrough region (bold), and recoded Ser (boxed) are highlighted.
(F) Identification of VEGFA mRNA readthrough product by MS/MS. A MS/MS spectrum was identified consistent with RSAGLEEGASLR (readthrough amino acid is underlined). The spectrum contains a total of nine C-terminal ‘y’ ions and seven N-terminal ‘b’ ions consistent with this sequence. The peptide was a low abundant component, and the spectrum contains several contaminant ions (*).
(G) Identity of readthrough peptide validated by synthetic peptide. RSAGLEEGASLR peptide was synthesized and the MS/MS spectrum determined as in (F).
See also Figure S2.