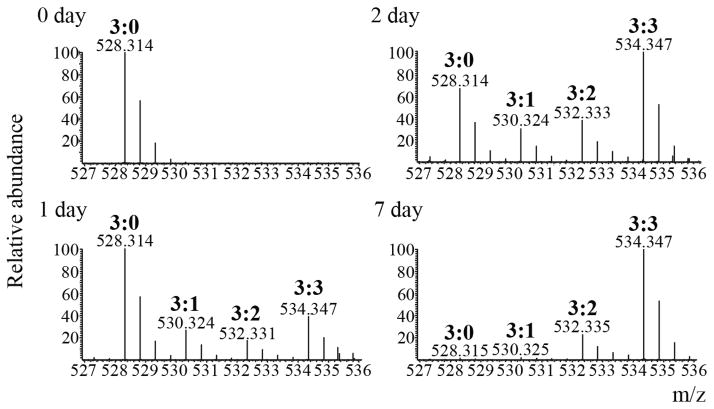
Figure 3.
Monitoring rate of H3.1K9me3. Full scan mass spectra of the [M+2H]2+ ions from the histone H3.1 peptide Kme3STGGKAPR extracted from the different time points (days 0, 1, 2 and 7) after pulse heavy methionine labeling. Intermediately labeled trimethylated forms (me3:1, 530.324 m/z and me3:2, 532.333 m/z) are present between the unlabeled trimethylated form (me3:0, 528.314 m/z) and the fully labeled trimethylated form (me3:3, 534.347 m/z) after day 0. Turnover of the old H3K9me3:0 can be seen in day 7, while the new H3K9me3:3 form is highest in abundance at that same time point.