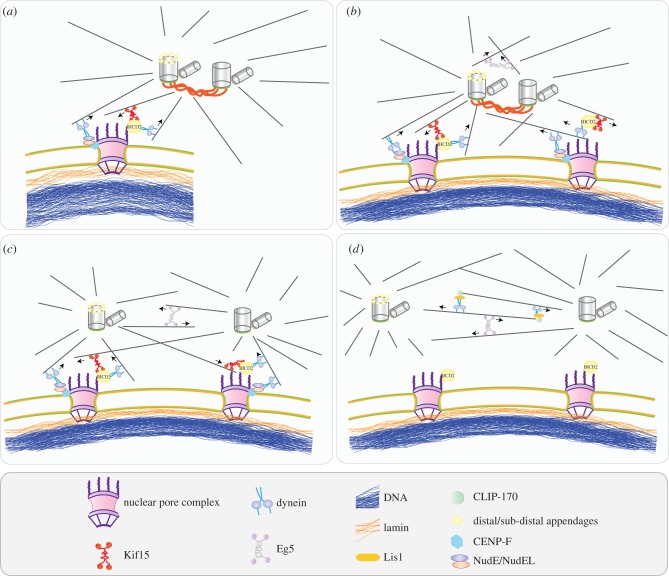
Figure 2.
Centrosome positioning around the nucleus. (a) In G2 phase, the mother centrosome is attached to the NPC. Two mechanisms account for this attachment. First, Nup358, a component of the NPC, minus-end directed motor protein dynein and the adaptor protein BICD2 form a complex which attaches to astral MTs emanating from the mother centrosome. Second, the N-terminal domain of Nup133 interacts with CENP-F that becomes positioned at the NE in G2/M. Dynein binds via the NudE/NudEL/CENP-F complex. Recruitment of BICD2 or CENP-F to the NPC does not depend on each other, which implies that the two pathways are independent from each other [45]. (b) When both centrosomes attach to the nucleus, the pull-and-push forces created by dynein and the kinesin motor Kif1 separate the centrosomes, and the centrosomes start to slide on the nucleus by being attached to the next NPC. (c) When they are separated enough to form anti-parallel MTs, dynein and the plus-end directed motor protein Eg5 become involved in further separation of the centrosomes. Dynein and Eg5 create opposing forces in order to balance the separation. Eg5 pushes the centrosomes away from each other, although dynein tries to keep them together by forming a complex with plus-end protein CLIP-170 and adaptor protein Lis1. Dynein localizes to MTs arising from one centrosome and catches the plus-end of MTs from the other centrosome. By pulling the MTs, dynein creates an inward force opposite to Eg5. (d) Prophase centrosome separation is completed when the separated centrosomes detach from the nucleus.