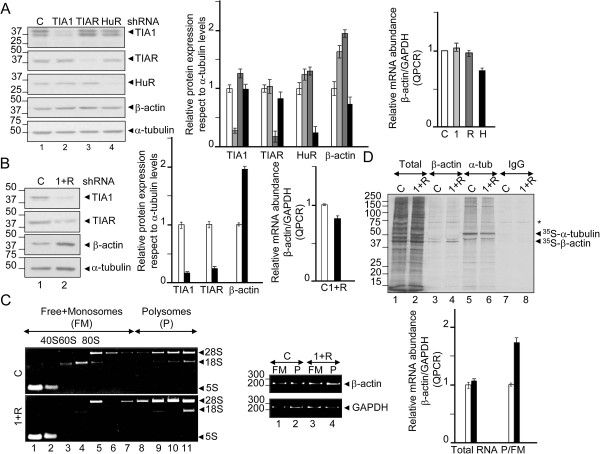
Figure 2.
Permanent TIA depletion improves β-actin mRNA translation. (A and B) Western blotting and reverse transcription-real time PCR (RT-QPCR) analysis showing knockdown efficiencies of control (c, white bars), TIA1 (1, lightgray bars), TIAR (R, darkgray bars), TIA1 plus TIAR (1 + R, black bars) and HuR (H, gray bars) and their effects on β-actin protein expression and mRNA levels. (C) Polysome profiling analysis using sucrose gradients from control (c, white bars) and TIA1 plus TIAR (1 + R, black bars)-knocked down HeLa cells. The quality of polysome preparation was verified by electrophoresis analysis in TBE-2% agarose gels of RNA content in each fraction. Ribosomal 5S, 18S and 28S bands are shown. Density distribution of relative translational efficiencies of β-actin and GAPDH mRNAs was verified by semiquantitative and QPCR analysis, respectively, in fraction pools identified as free-monosomes (FM) (fractions 1-7) and polysomes (fractions 8-11). Data are the mean ± SEM from two independent analysis. The represented values (A-C) were normalized and are expressed relative to control (c), whose value is fixed arbitrarily to 1. (D) Nascent translation of total, β-actin and α-tubulin proteins was determined by incubation of control (c) and TIA1 + TIAR (1 + R)-silenced HeLa cells in the presence of 35S-methionine/cystein mix. After immunoprecipitation using anti-β-actin, anti-α-tubulin and IgG (negative control) antibodies, the newly translated proteins were visualized by 10% SDS-PAGE and autoradiography. In all indicated cases, molecular weight markers for protein or DNA and the identities of proteins and amplified PCR products are shown.