Abstract
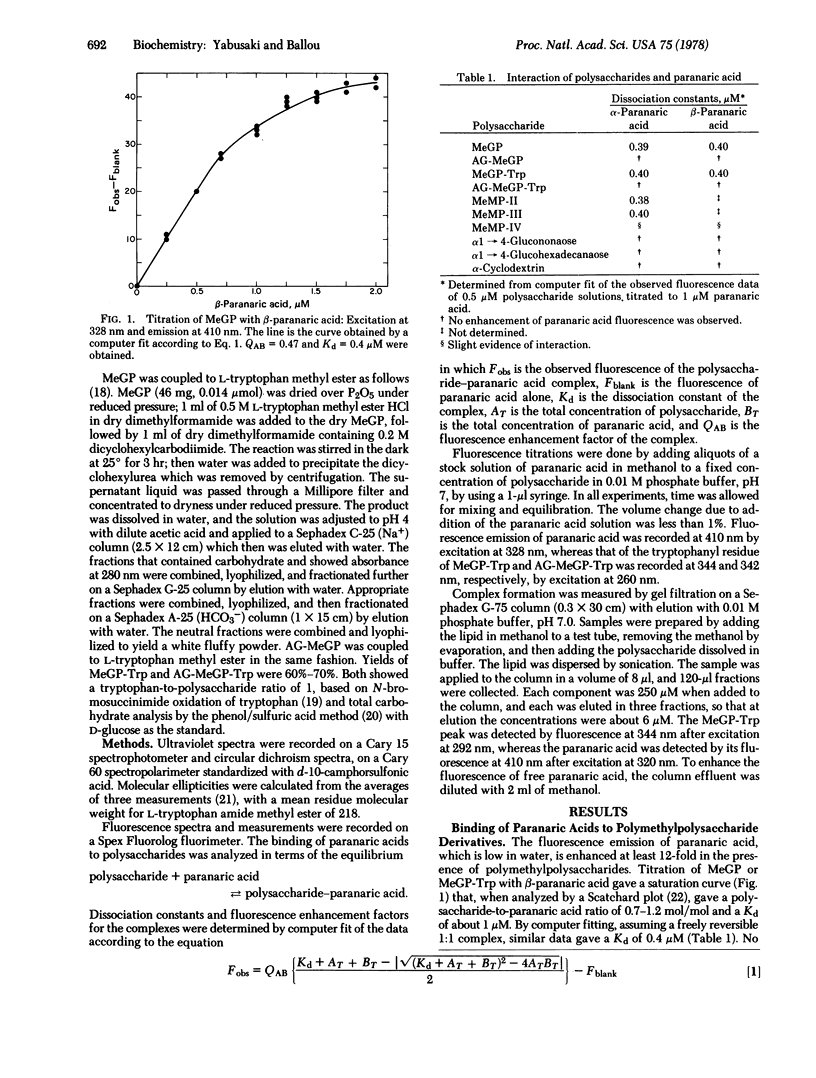
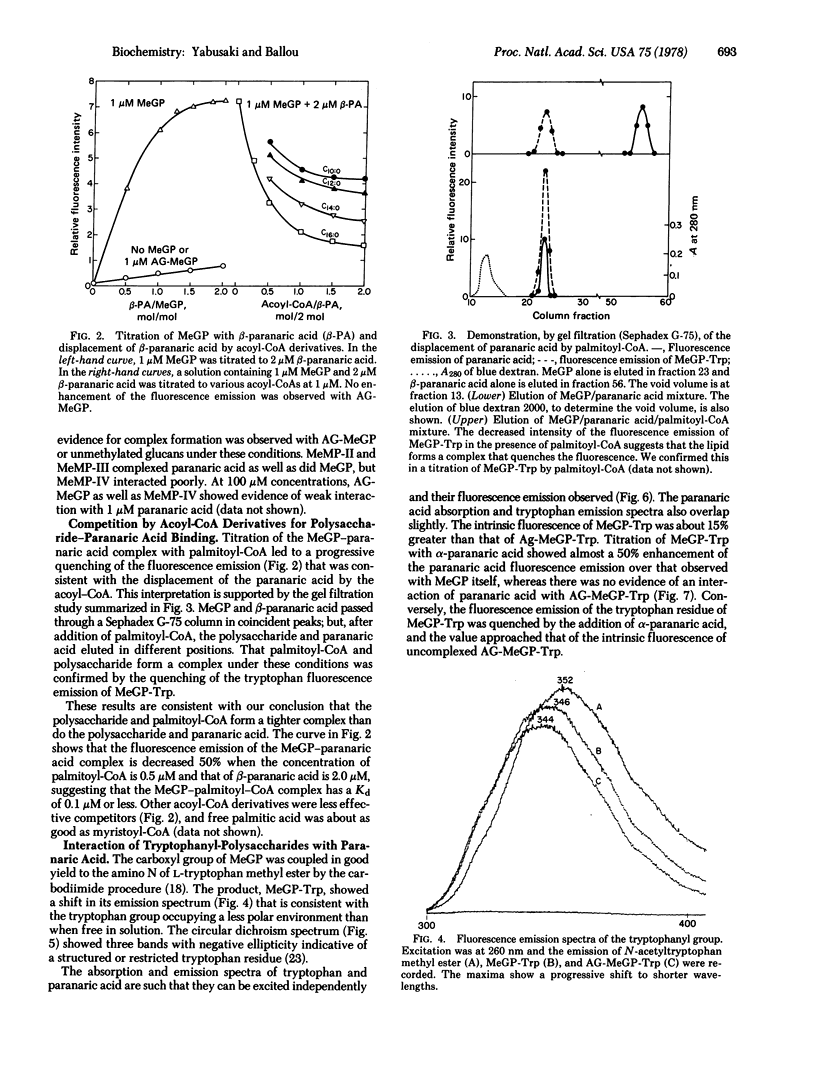
The long-chain polyenoic fatty acids α- and β-paranaric acid form complexes with the 6-O-methylglucose polysaccharide from Mycobacterium smegmatis as demonstrated by an enhanced fluorescence emission of the paranaric acid. This interaction is eliminated by digestion of the methylglucose polysaccharide with α-amylase and glucoamylase, which removes four hexose units from the nonreducing end of the chain. Titration of the methylglucose polysaccharide with either paranaric acid isomer suggests formation of a 1:1 complex with a dissociation constant (Kd) of 0.4 μM. The fluorescence emission of this complex is quenched by palmitoyl-CoA, which indicates that the paranaric acid can be displaced by the acoyl-CoA, a conclusion confirmed by gel filtration. The presumed polysaccharide/palmitoyl-CoA complex has a Kd of about 0.1 μM. Acoyl-CoA derivatives with shorter fatty acid chains and free palmitic acid complete less effectively, indicating that they form weaker complexes with the polysaccharide. The methylmannose polysaccharides with 12 or 13 sugar units also complex paranaric acid strongly (Kd ∼ 0.4 μM), whereas the isomer with 11 sugar units complexes weakly.
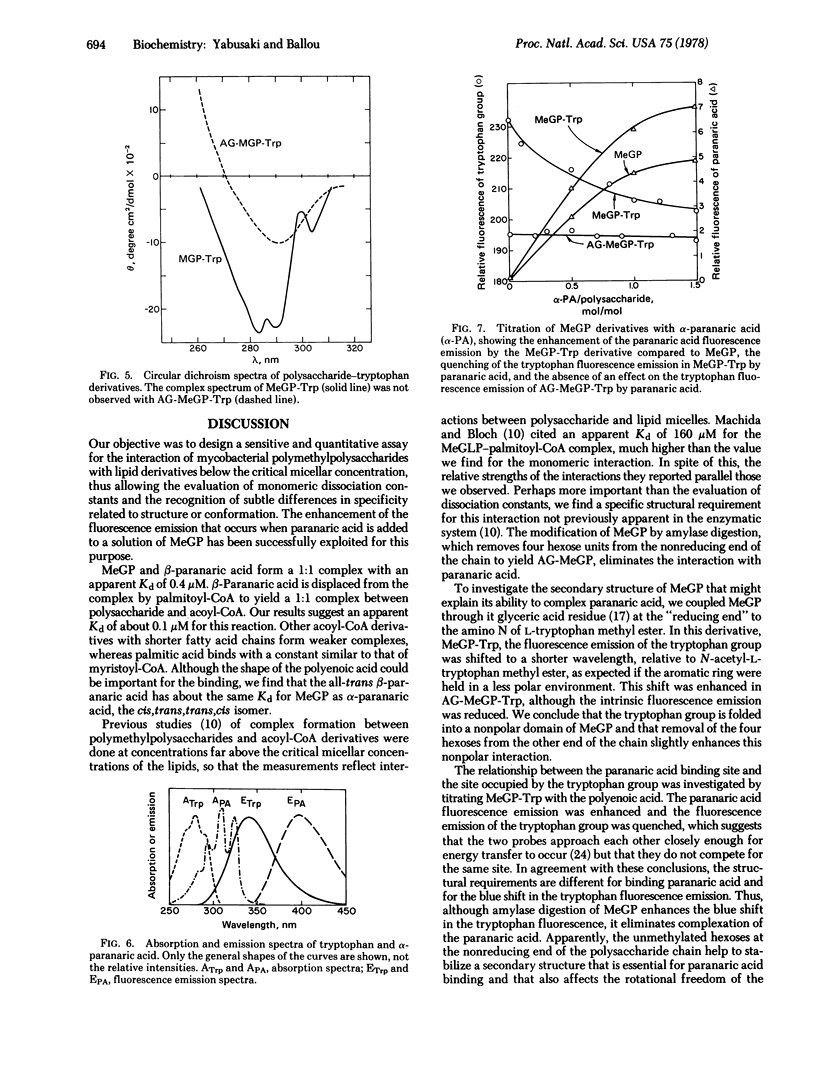
The methylglucose polysaccharide has been coupled to L-tryptophan methyl ester. The fluorescence emission spectrum of the attached tryptophan group is shifted to a shorter wavelength relative to N-acetyl-L-tryptophan methyl ester, and this effect is enhanced in the corresponding derivative made with the amylase-digested polysaccharide. The circular dichroism spectrum of the polysaccharide—tryptophan derivative shows three bands with negative ellipticity, in the 270-300 nm region, not observed in the amylase-digested derivative. These results imply that the tryptophan is in a more structured environment in the former than in the latter derivative. α-Paranaric acid binds to the polysaccharide—tryptophan conjugate and shows an enhanced fluorescence emission with partial quenching of the tryptophan fluorescence emission, suggestive of Förster energy transfer from tryptophan to paranaric acid.
Keywords: polysaccharide—lipid interaction, fluorescence, circular dichroism, tryptophan, fatty acid synthetase
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Flick P. K., Bloch K. Vitro alterations of the product distribution of the fatty synthetase from Mycobacterium phlei. J Biol Chem. 1974 Feb 25;249(4):1031–1036. [PubMed] [Google Scholar]
- Gray G. R., Ballou C. E. Isolation and characterization of a polysaccharide containing 3-O-methyl-D-mannose from Mycobacterium phlei. J Biol Chem. 1971 Nov 25;246(22):6835–6842. [PubMed] [Google Scholar]
- Ilton M., Jevans A. W., McCarthy E. D., Vance D., White H. B., 3rd, Bloch K. Fatty acid synthetase activity in Mycobacterium phlei: regulation by polysaccharides. Proc Natl Acad Sci U S A. 1971 Jan;68(1):87–91. doi: 10.1073/pnas.68.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller J., Ballou C. E. The 6-O-methylglucose-containing lipopolysaccharide of Mycobacterium phlei. Identification of the lipid components. J Biol Chem. 1968 Jun 10;243(11):2905–2910. [PubMed] [Google Scholar]
- Knoche H., Esders T. W., Koths K., Bloch K. Palmityl coenzyme A inhibition of fatty acid synthesis. Relief by bovine serum albumin and mycobacterial polysaccharides. J Biol Chem. 1973 Apr 10;248(7):2317–2322. [PubMed] [Google Scholar]
- Machida Y., Bloch K. Complex formation between mycobacterial polysaccharides and fatty acyl-CoA derivatives. Proc Natl Acad Sci U S A. 1973 Apr;70(4):1146–1148. doi: 10.1073/pnas.70.4.1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maitra S. K., Ballou C. E. Heterogeneity and refined structtures of 3-O-methyl-D-mannose polysaccharides from Mycobacterium smegmatis. J Biol Chem. 1977 Apr 25;252(8):2459–2469. [PubMed] [Google Scholar]
- Saier M. H., Jr, Ballou C. E. The 6-O-methylglucose-containig lipopolysaccharide of Mycobacterium phlei. Complet structure of the polysaccharide. J Biol Chem. 1968 Aug 25;243(16):4332–4341. [PubMed] [Google Scholar]
- Saier M. H., Jr, Ballou C. E. The 6-O-methylglucose-containing lipopolysaccharide of Mycobacterium phlei. Identification of D-glyceric acid and 3-O-methyl-D-glucose in the polysaccharide. J Biol Chem. 1968 Mar 10;243(5):992–1005. [PubMed] [Google Scholar]
- Sklar L. A., Hudson B. S., Petersen M., Diamond J. Conjugated polyene fatty acids on fluorescent probes: spectroscopic characterization. Biochemistry. 1977 Mar 8;16(5):813–819. doi: 10.1021/bi00624a001. [DOI] [PubMed] [Google Scholar]
- Sklar L. A., Hudson B. S., Simoni R. D. Conjugated polyene fatty acids as membrane probes: preliminary characterization. Proc Natl Acad Sci U S A. 1975 May;72(5):1649–1653. doi: 10.1073/pnas.72.5.1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith W. L., Ballou C. E. The 6-O-methylglucose-containing lipopolysaccharides of Mycobacterium phlei. Locations of the neutral and acidic acyl groups. J Biol Chem. 1973 Oct 25;248(20):7118–7125. [PubMed] [Google Scholar]
- Strickland E. H., Horwitz J., Billups C. Fine structure in the near-ultraviolet circular dichroism and absorption spectra of tryptophan derivatives and chymotrypsinogen A at 77 degrees K. Biochemistry. 1969 Aug;8(8):3205–3213. doi: 10.1021/bi00836a012. [DOI] [PubMed] [Google Scholar]
- Wood W. I., Peterson D. O., Bloch K. Mycobacterium smegmatis fatty acid synthetase. A mechanism based on steady state rates and product distributions. J Biol Chem. 1977 Aug 25;252(16):5745–5749. [PubMed] [Google Scholar]


