Abstract
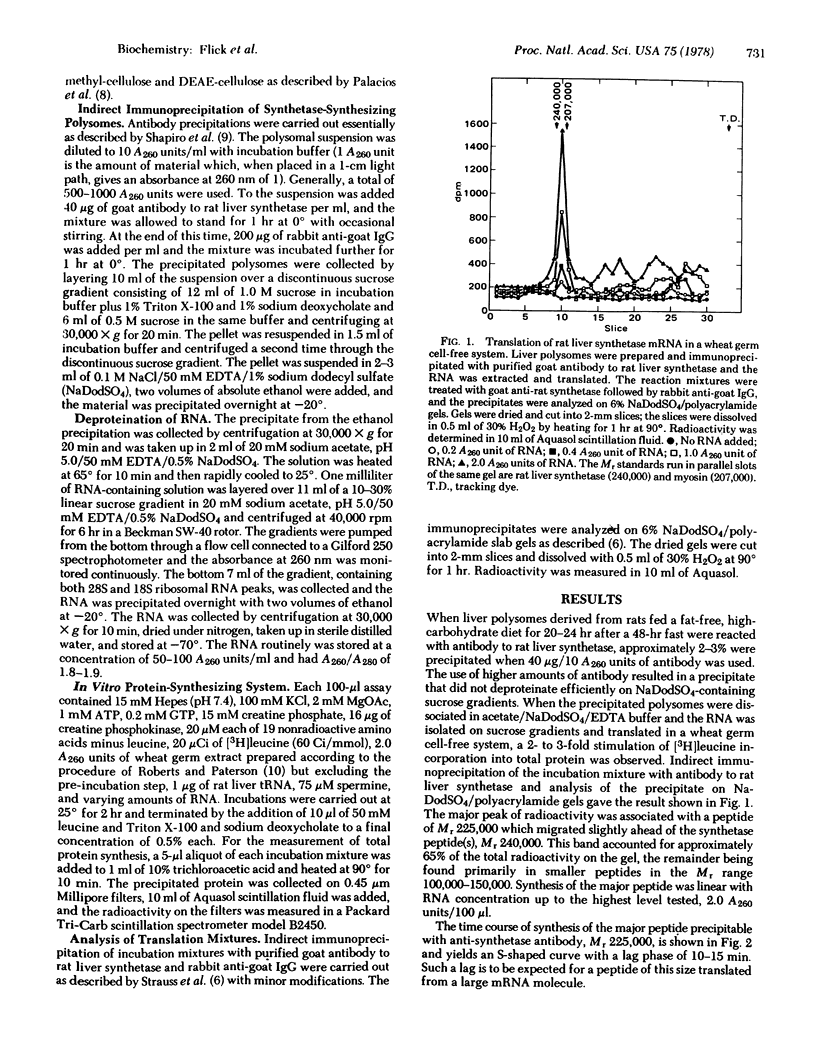
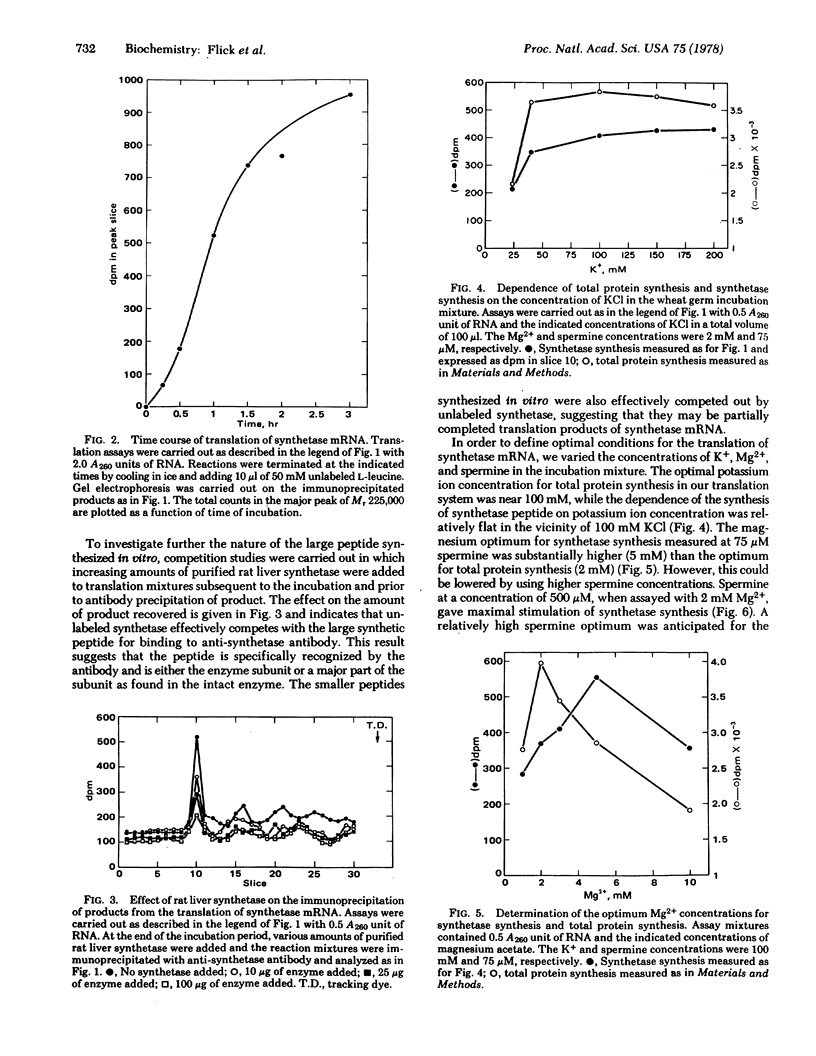
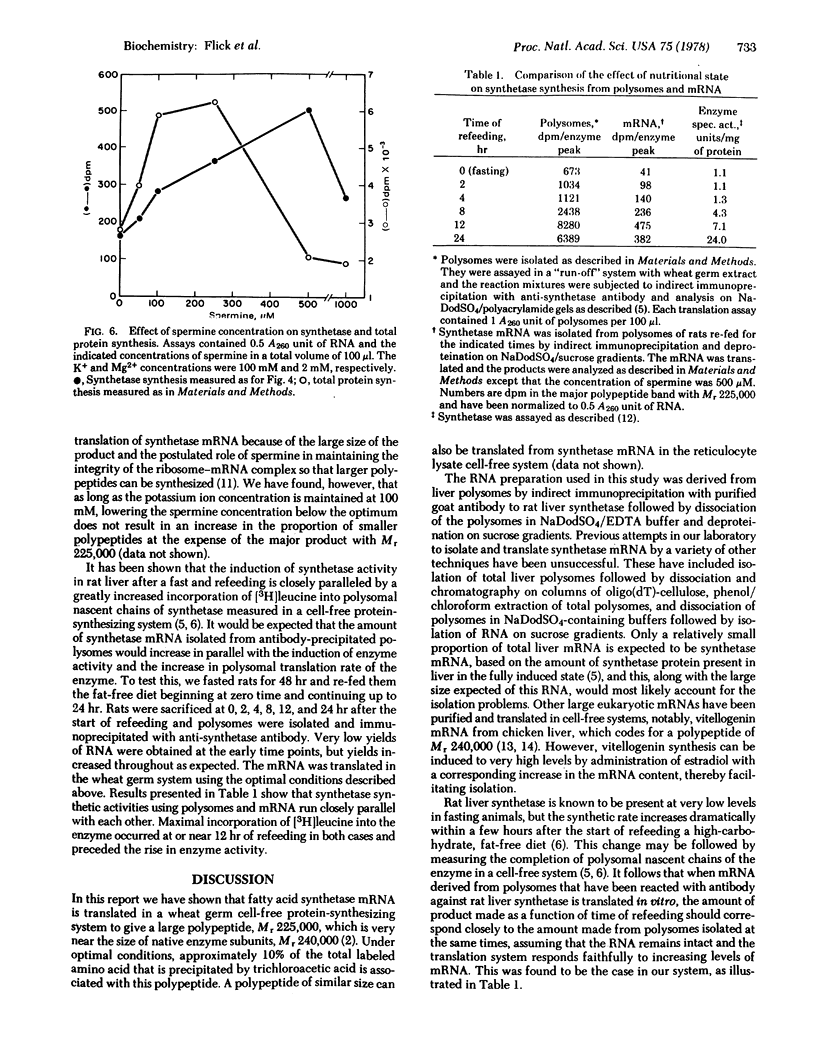
Total liver polysomes were isolated from rats that had fasted for 48 hr and that then had been re-fed a high-carbohydrate, fat-free diet for 20-24 hr. Indirect immunoprecipitation of the polysomes with purified antibody to rat liver fatty acid synthetase and deproteination on sodium dodecyl sulfate-containing sucrose gradients gave an RNA fraction which, when translated in a cell-free system derived from wheat germ, yielded a major polypeptide of apparent molecular weight 225,000 when the translation products were analyzed by polyacrylamide gel electrophoresis in sodium dodecyl sulfate. The polypeptide was specifically precipitated with antibody against rat liver fatty acid synthetase and competed with unlabeled fatty acid synthetase for binding to the antibody. It was somewhat smaller than native fatty acid synthetase subunits (molecular weight 240,000). The peptide accounted for approximately 65% of the radioactive, antibody-precipitable product, the remainder being peptides in the molecular weight range 100,000-150,000. Synthesis of the polypeptide was optimized with respect to K+, Mg2+, and spermine concentrations. The quantity of fatty acid synthetase mRNA obtained by the above procedure and measured by translation was a function of the nutritional state of the animal. The relative activity in fasting rats compared to rats that were re-fed for 12 hr was 1:12. The data suggest that rat liver fatty acid synthetase is synthesized as intact subunits from a large mRNA molecule or molecules.
Keywords: mRNA translation, wheat germ cell-free system, regulation of fatty acid synthetase
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alberts A. W., Ferguson K., Hennessy S., Vagelos P. R. Regulation of lipid synthesis in cultured animal cells. J Biol Chem. 1974 Aug 25;249(16):5241–5249. [PubMed] [Google Scholar]
- Alberts A. W., Strauss A. W., Hennessy S., Vagelos P. R. Regulation of synthesis of hepatic fatty acid synthetase: binding of fatty acid synthetase antibodies to polysomes. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3956–3960. doi: 10.1073/pnas.72.10.3956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton D. N., Collins J. M., Kennan A. L., Porter J. W. The effects of nutritional and hormonal factors on the fatty acid synthetase level of rat liver. J Biol Chem. 1969 Aug 25;244(16):4510–4516. [PubMed] [Google Scholar]
- Flick P. K., Chen J., Vagelos P. R. Effect of dietary linoleate on synthesis and degradation of fatty acid synthetase from rat liver. J Biol Chem. 1977 Jun 25;252(12):4242–4249. [PubMed] [Google Scholar]
- Hunter A. R., Farrell P. J., Jackson R. J., Hunt T. The role of polyamines in cell-free protein synthesis in the wheat-germ system. Eur J Biochem. 1977 May 2;75(1):149–157. doi: 10.1111/j.1432-1033.1977.tb11512.x. [DOI] [PubMed] [Google Scholar]
- Jost J. P., Pehling G. Immunochemical isolation and characterization of vitellogenin mRNA from liver of estradiol-treated chicks. Eur J Biochem. 1976 Jul 1;66(2):339–346. doi: 10.1111/j.1432-1033.1976.tb10523.x. [DOI] [PubMed] [Google Scholar]
- Jost J. P., Pehling G. Organization of vitellogenin polysomes, size of the mRNA and polyadenylate fragment. Eur J Biochem. 1976 Feb 16;62(2):299–306. doi: 10.1111/j.1432-1033.1976.tb10161.x. [DOI] [PubMed] [Google Scholar]
- Palacios R., Sullivan D., Summers N. M., Kiely M. L., Schimke R. T. Purification of ovalbumin messenger ribonucleic acid by specific immunoadsorption of ovalbumin-synthesizing polysomes and millipore partition of ribonucleic acid. J Biol Chem. 1973 Jan 25;248(2):540–548. [PubMed] [Google Scholar]
- Palmiter R. D. Magnesium precipitation of ribonucleoprotein complexes. Expedient techniques for the isolation of undergraded polysomes and messenger ribonucleic acid. Biochemistry. 1974 Aug 13;13(17):3606–3615. doi: 10.1021/bi00714a032. [DOI] [PubMed] [Google Scholar]
- Roberts B. E., Paterson B. M. Efficient translation of tobacco mosaic virus RNA and rabbit globin 9S RNA in a cell-free system from commercial wheat germ. Proc Natl Acad Sci U S A. 1973 Aug;70(8):2330–2334. doi: 10.1073/pnas.70.8.2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro D. J., Taylor J. M., McKnight G. S., Palacios R., Gonzalez C., Kiely M. L., Schimke R. T. Isolation of hen oviduct ovalbumin and rat live albumin polysomes by indirect immunoprecipitation. J Biol Chem. 1974 Jun 25;249(12):3665–3671. [PubMed] [Google Scholar]
- Smith S. Studies on the immunological cross-reactivity and physical properties of fatty acid synthetases. Arch Biochem Biophys. 1973 Jun;156(2):751–758. doi: 10.1016/0003-9861(73)90328-7. [DOI] [PubMed] [Google Scholar]
- Strauss A. W., Alberts A. W., Hennessy S., Vagelos P. R. Regulation of synthesis of hepatic fatty acid synthetase: polysomal translation in a cell-free system. Proc Natl Acad Sci U S A. 1975 Nov;72(11):4366–4370. doi: 10.1073/pnas.72.11.4366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma I. M., Temple G. F., Fan H., Baltimore D. In vitro synthesis of DNA complementary to rabbit reticulocyte 10S RNA. Nat New Biol. 1972 Feb 9;235(58):163–167. doi: 10.1038/newbio235163a0. [DOI] [PubMed] [Google Scholar]
- Volpe J. J., Vagelos P. R. Mechanisms and regulation of biosynthesis of saturated fatty acids. Physiol Rev. 1976 Apr;56(2):339–417. doi: 10.1152/physrev.1976.56.2.339. [DOI] [PubMed] [Google Scholar]


