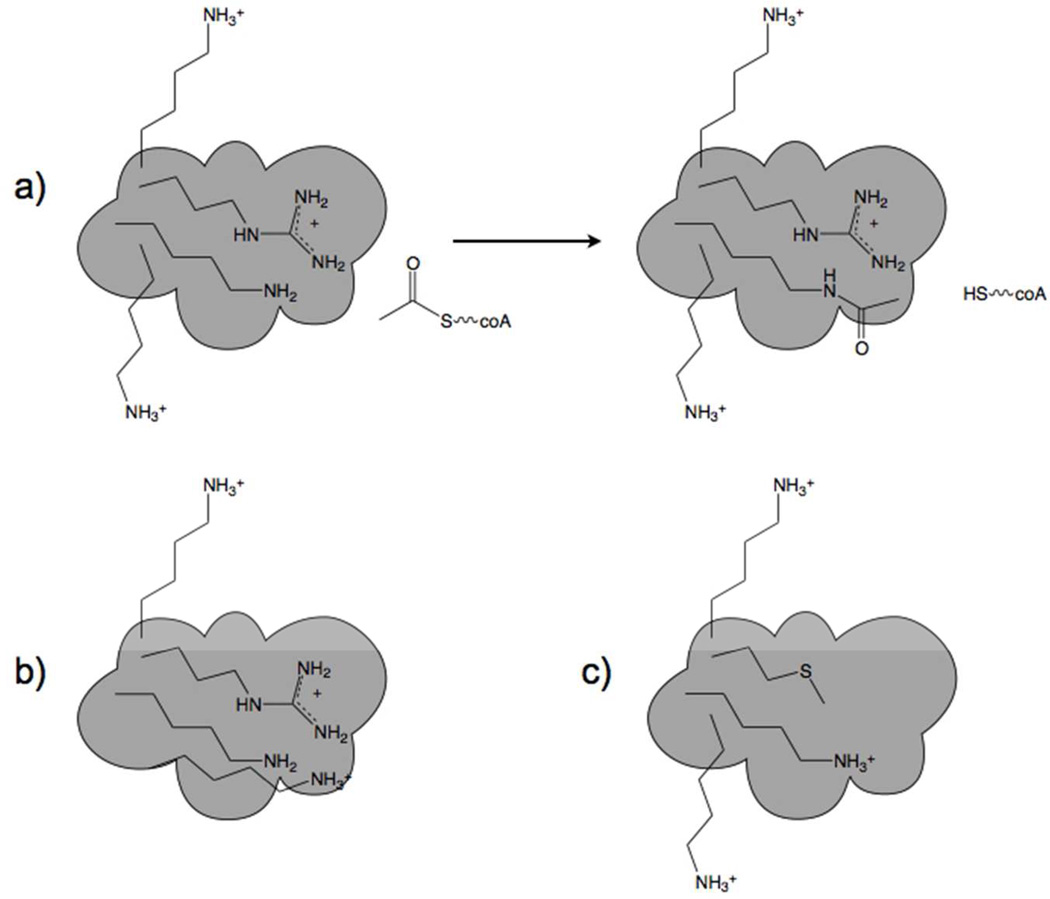
Figure 3.
Chemistry and mutability of acetylation. a) Near neutral pH, Lys residues with nonperturbed pKa values are resistant to acetylation. One Lys near an Arg has a depressed pKa value and is modified by Ac-coA. b) A susceptible Lys residue has its pKa value further depressed by virtue of accumulation of an additional nearby positive charge. This is the postulated mechanism by which regulatory Lys residues frequently acquired susceptibility to chemical acetylation. Largely, mitochondrial enzyme inactivation is proposed to be under positive selective pressure by favoring lipogenesis. c) Here a formerly accessible Lys residue acquires greater resistance to acetylation by virtue of loss of a nearby positive charge. This adaptive mechanism would protect against loss of functions that are essential, particularly during times of nutritional oversufficiency or undersufficiency.