Figure 2.
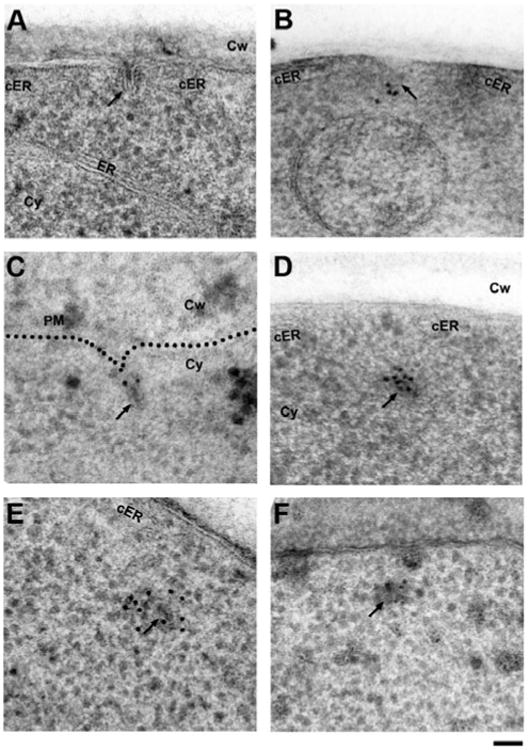
Establishing freeze-substitution and low-temperature embedding conditions for anti-GFP immunolabeling of endocytic proteins in S. cerevisiae. A: Freeze-substitution in acetone containing 2% water and 0.1% uranyl acetate followed by washes in pure acetone and embedding in HM20 results in excellent structural preservation of typical intermediate endocytic sites (arrow), but anti-GFP immunolabeling fails. Note the cortical ER cisternae (cER) flanking the endocytic site. B: Maintaining hydration of GFP by including 2% water in all acetone solutions (washes and infiltration) allows detection of the coat protein Sla1p-GFP at the tip of endocytic membranes (arrow) with a commercial primary anti-GFP antibody (Fitzgerald Industries), which was used throughout the study, except for panels E and F. C: Labeling of a strain expressing the BAR protein Rvs167p-GFP detects the protein at the neck region as previously described (Idrissi et al., 2008). D: The same antibody intensely labels the primary endosome (arrow) in a strain expressing Abp1p-GFP (see background quantification in Supplementary Table 1). The opening of the cortical ER (cER) is transiently maintained after scission. E: Labeling of the same Abp1p-GFP strain with a different, previously published anti-GFP antibody (Seedorf et al., 1999) also labels the post-scission primary endosomes (arrow). F: Identical but less efficient labeling of Abp1p-GFP using a different antibody (Collins et al., 2005). ER, endoplasmic reticulum; cER, cortical endoplasmic reticulum; Cy, cytoplasm; Cw, cell wall. Scale bar = 50 nm.
