Fig. 5.
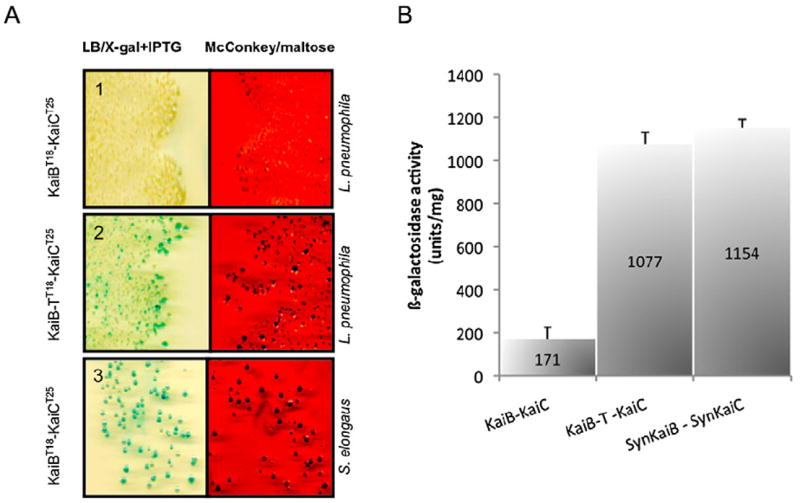
Bacterial two hybrid assay suggests that Legionella pneumophila KaiC and KaiB do not interact. The interaction test is based on the reconstitution of the adenylate cyclase activity in an Escherichia coli strain lacking endogenous adenylate cyclase.
A. KaiC and KaiB from L. pneumophila and Synechococcus elongatus were fused to T25 and T18-domain of the adenylate cyclase respectively. Interaction assay of KaiC with KaiB of L. pneumophila (Panel 1). Interaction assay KaiC with KaiB-T in which the C-terminal residues from T. elongatus are fused to L. pneumophila KaiB (Panel 2). Interaction assay with, KaiC from S. elongatus and KaiB from L. pneumophila used as a positive control showed interaction on the indicator media (Panel 3).
B. The β-galactosidase activity was measured for each combination of proteins. KaiB-T and KaiC from Legionella pneumophila display similar levels of β -galactosidase suggesting the importance of the C-terminal residues of KaiB for interaction with KaiC. The bars represent the mean values of three independent experiments ± standard deviation.
