Figure 1. Antibody structure and sequence diversification mechanisms.
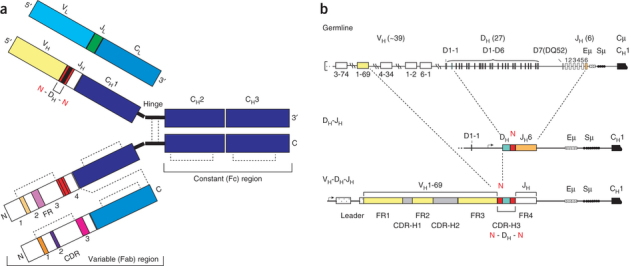
(a) Schematic of IgG structure. In the top chains, domains encoded from germline V, D, J and C segments are indicated. Nontemplated N-nucleotides are shown in red. These top chains delineate the 5′ to 3′ genetic composition of the antibody. In the bottom chains, framework (FR) and complementarity-determining regions (CDRs) are indicated. These bottom chains delineate the N-terminal to C-terminal protein sequence. Dashed lines denote disulfide bonds. (b) Key steps in antibody diversification. The primary antibody heavy chain repertoire is created predominantly by the somatic recombination of variable (V), diversity (D) and joining (J) gene segments, and by the random nontemplated addition of N-nucleotides. The antigen-binding site of a heavy chain is formed by the juxtaposition of the hypervariable complementarity-determining regions (CDR-H1, H2 and H3) and the framework 3 region (FR3). After productive IgH rearrangement, recombination of the light chain (IgL) ensues, and the heterodimeric pairing of H and L chains forms the complete antibody of the IgM isotype that is expressed on the surface of a newly formed immature B cell. Eμ: IgM intronic enhancer; Sμ: tandem repeats critical for class-switch recombination. Numbers in parentheses refer to estimates of human germline VH DH and JH segments.
