Figure 2. Key steps in the development of antigen-specific B cells.
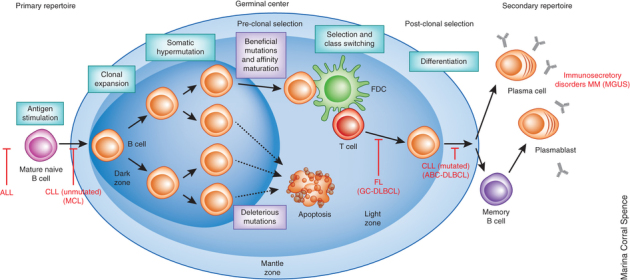
The steps of normal B-cell differentiation and diversification of the antibody repertoire are indicated in black text. Normal B cells are generated in the bone marrow, migrate to the periphery and, following developmental checkpoint selection, comprise the population of IgM+IgD+ mature naive B cells. When these cells are activated by cognate antigen in the presence of T-cell help, they enter a germinal center (GC) reaction where they rapidly proliferate; this results in clonal expansion and subsequent somatic hypermutation catalyzed by activation-induced cytidine deaminase. B cells bearing antibodies with high affinity for cognate antigen and that survive the GC reaction can undergo class-switch recombination to IgG, IgA or IgE isotypes and ultimately differentiate into memory B cells, antibody-secreting plasmablasts or plasma cells. After subsequent encounter with the same cognate antigen, memory B cells can proliferate or differentiate directly into antibody-secreting cells. Steps that proceed abnormally, leading to the development of human B-cell leukemias and lymphomas, are indicated in red text. ALL, acute lymphoblastic leukemia; CLL, chronic lymphocytic leukemia; MCL, mantle cell lymphoma; GC-DLBCL, germinal center diffuse large B cell lymphoma; FL, follicular lymphoma; ABC-DLBCL, activated B cell–like DLBCL; MGUS, monoclonal gammopathy of undetermined significance; MM, multiple myeloma. B-cell malignancies that have not been analyzed extensively by high-throughput sequencing are shown in parenthesis.
