Fig. 1.
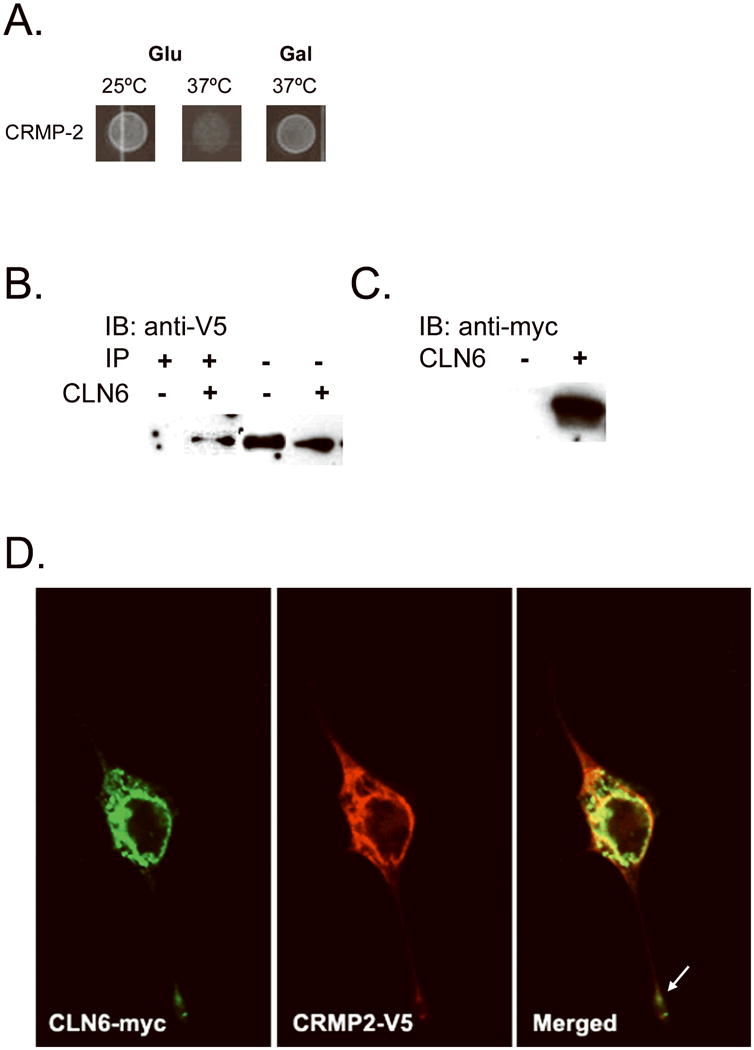
CLN6 interacts with collapsin response mediator protein-2 (CRMP-2). CLN6 hydrophilic fragments fused to the human Sos protein were screened using the Cytotrap yeast two-hybrid (Y2H) method against a human fetal brain library to identify interacting partners. The library proteins were expressed when grown on galactose media; thus, positive interaction was achieved when there is growth on galactose at the permissive temperature of 37°C. We found that CLN6 interacted with CRMP-2 (A). To validate the CRMP-2 interaction, CLN6-myc and CRMP-2-V5 were coexpressed or CRMP-2 alone was expressed in NIH/3T3 fibroblasts, and protein lysates were immunoprecipitated with anti-myc antibody. The immunoprecipitate (B, left) and the lysate (B, right) were probed with an anti-V5 antibody. This showed a specific CRMP-2 band in the immunoprecipitation only in the presence of CLN6. The lysate was probed with an anti-myc antibody to verify CLN6 expression (C). To visualize intracellular localization, CLN6-myc and CRMP-2-V5 were transfected into NIH/3T3 fibroblasts grown on poly-d-lysine-treated cover glass. Anti-myc and Anti-V5 antibodies were used to stain CLN6-myc (D, left) and CRMP-2-V5 (D, middle), respectively. Alexa-fluor antibodies were used to visualize the proteins. Confocal microscopy was used to image the cells. Colocalization of the two proteins is shown by the yellow color in the merged image (D, right). The white arrow indicates costaining down a process (magnification 40×).
