Insight from Michael Heneka
Macrophages mediate myelin destruction in multiple sclerosis (MS), but the origin of these cells (whether derived from tissue-resident microglial cells or infiltrating monocytes) has been widely debated. Now, Yamasaki and colleagues distinguish these cells in a mouse model of MS and show that monocyte-derived macrophages (MDMs) mediate myelin destruction, whereas microglia-derived macrophages (MiDMs) clear up the debris.
Previous attempts to decipher the nature and role of cells involved in autoimmune demyelination have proven challenging. Although ontogenetically distinct, it has not been possible to distinguish macrophages derived from tissue-resident or -infiltrating cells based on morphological features (by light microscopy) or surface phenotype. Previous attempts to address this problem include parabiosis and bone marrow transplantation after irradiation, both strategies with substantial technical problems and limitations.
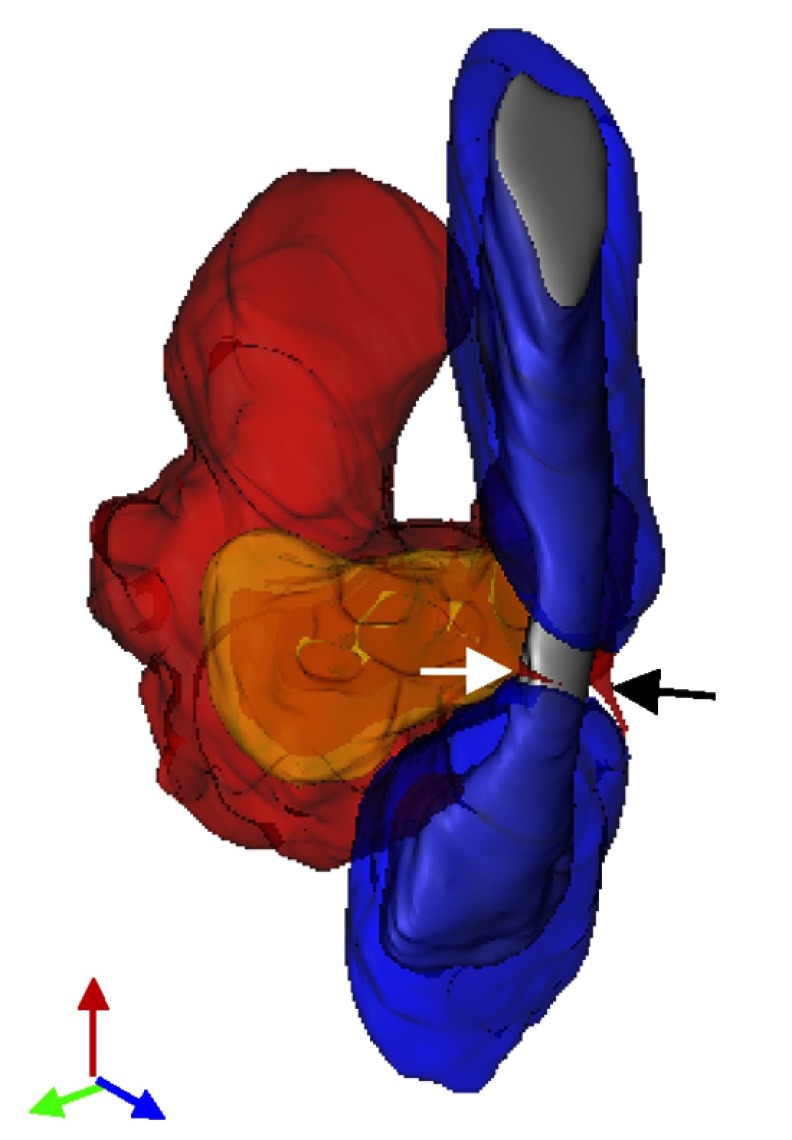
Nodes of Ranvier represent a prime site of attack for MDMs at the onset of EAE. This 3D reconstruction of SBF-SEM images shows a monocyte-derived macrophage encircling the node of Ranvier, as shown by the two primary processes (white and black arrows).
Yamasaki et al. studied double chemokine receptor (CCR2-RFP+; CX3CR1-GFP+) mice in the experimental autoimmune encephalomyelitis (EAE) mouse model of MS. Inflammatory lesions were filled with both MDMs and MiDMs. Confocal immunohistochemistry, serial block-face scanning electron microscopy (SBF-SEM), and subsequent 3D reconstruction revealed that myelin destruction was initiated by MDMs, often at the nodes of Ranvier, whereas MiDMs were not detected at this site. Disruption of MDM infiltration by CCR2 deficiency completely abolished the presence of macrophages at the nodes of Ranvier. Gene expression profiling of both cell types at disease onset revealed substantial differences, which correlated well with the observations obtained by SBF-SEM. MDMs expressed genes attributable to effector functions, including those involved in phagocytosis and cell clearance. In contrast, MiMD gene expression patterns at disease onset were characteristic of a repressed metabolic state.
This paper sets a new standard for further studies in the field. For the first time, MDMs and MiDMs have been clearly differentiated and their morphological relationship to axoglial structures has been analyzed. The finding that MDMs rather than MiDMs initiate myelin destruction at disease onset should enable this cell population to be targeted more effectively in future. The next stage is to verify these findings in human tissue. Future research should also assess further time points over the entire disease course, in particular to exclude that MiDMs do not join MDMs at the node of Ranvier at later stages of disease. A precise distinction between local and infiltrating cell populations may also contribute to a better understanding of pathogenesis in other CNS disorders such as stroke and brain trauma and will hopefully lead to the development of new therapeutic strategies.
References
- Yamasaki, R., et al. 2014. J. Exp. Med. 10.1084/jem.20132477. [DOI] [Google Scholar]