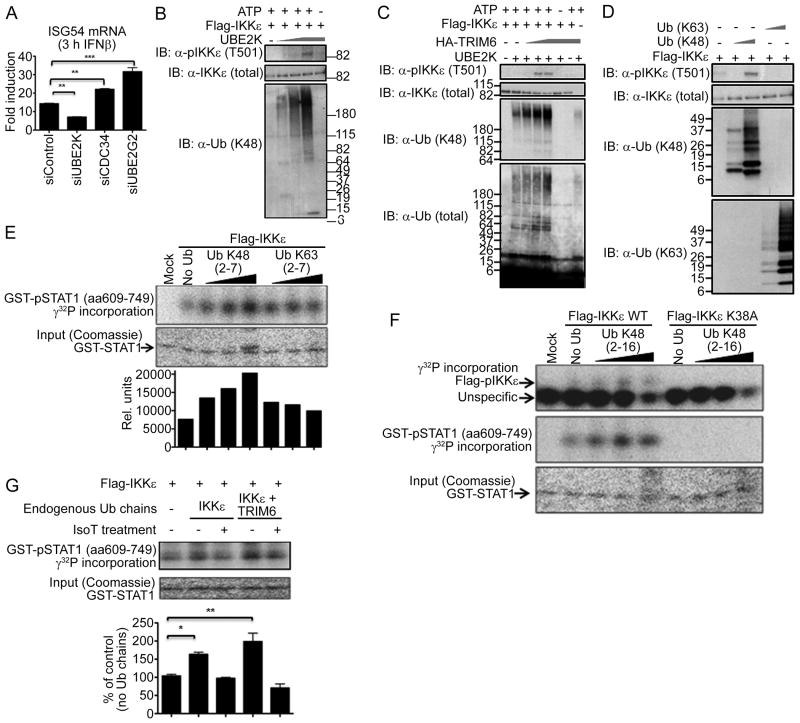
Figure 7. The E2-ligase UbE2K and TRIM6 catalyze K48-linked ubiquitin chains that activate IKKε for STAT1 phosphorylation in vitro.
(A) Knockdown of endogenous E2 conjugases UbE2K, CDC34 and UbE2G2 in A549 cells. At 40 h p.t., cells were mock-treated or stimulated with IFNβ (500 U/ml) for 3 h and cells were harvested for RT-qPCR analysis. Values are depicted as fold induction over non-stimulated cells. (B) Purified FLAG-IKKε was incubated with recombinant E1, UbE2K (0.025-0.5 μM), mono-Ub and ATP. Activation of IKKε was assessed by phophorylation of T501. (C) Increasing amounts of TRIM6 were added to limiting amounts of UbE2K (0.025 μM). (D) Recombinant K48 or K63-linked poly-Ub chains (2-7 Ub molecules/chain ) were incubated with FLAG-IKKε and subjected to IB analysis. (E-F) In vitro STAT1 kinase assay. Recombinant K48 or K63-linked poly-Ub chains (2-7 Ub/chain; (E), or K48 2-16 Ub/chain (F)) were incubated with purified IKKε WT (E and F) or an IKKε kinase-dead mutant (K38A; (F)) STAT1 and γ32ATP. The reaction mixture was subjected to SDS-PAGE and visualized by autoradiography. The bands were quantified using ImageJ software. (G) Endogenous unanchored poly-Ub chains induce IKKε-mediated STAT1 phosphorylation. WCE of HEK-293T cells, transfected with FLAG-IKKε and HA-TRIM6 were subjected to FLAG-IP. Poly-Ub chains bound to IKKε were isolated by incubation at 75 °C for 5min, followed by treatment with IsoT. These Ub chains were used for IKKε-mediated STAT1 kinase assay. The bands were quantified using ImageJ software, normalized by the input (Coomassie), and expressed as percentage of control without Ub chains. The quantification data are from 2 independent experiments. See also Figure S7.